860815P
Avanti
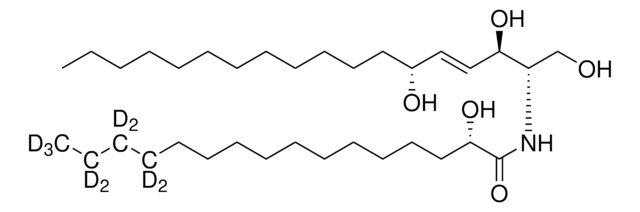
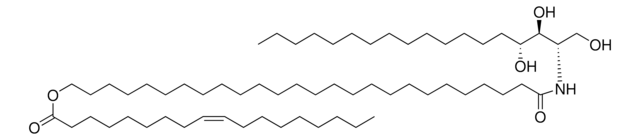
16:0(2R-OH) Ceramide
Avanti Research™ - A Croda Brand 860815P, powder
Synonym(s):
N-(2′-(R)-hydroxypalmitoyl)-D-erythro-sphingosine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C34H67NO4
CAS Number:
Molecular Weight:
553.90
UNSPSC Code:
12352211
NACRES:
NA.25
Recommended Products
form
powder
packaging
pkg of 1 × 25 mg (860815P-25mg)
pkg of 1 × 5 mg (860815P-5mg)
manufacturer/tradename
Avanti Research™ - A Croda Brand 860815P
lipid type
sphingolipids
shipped in
dry ice
storage temp.
−20°C
SMILES string
CCCCCCCCCCCCC/C=C/[C@@H](O)[C@@H](NC([C@H](O)CCCCCCCCCCCCCC)=O)CO
General description
Ceramides with 2-hydroxy fatty acids (hFA) group are present in the epidermis. 16:0(2R-OH) Ceramide is a unique ceramide containing 16C long chain base fatty acid (palmitic acid)-with 2′-hydroxyl group in R configuration.
Biochem/physiol Actions
Hydroxy fatty acid (hFA)-sphingolipids are implicated in cell signaling. They may also help in regulation of cell differentiation and apoptosis. Synthetic 2′R-hydroxy-C16-ceramide exhibits high proapoptotic activity than proapoptotic 2′S-hydroxy-C16-ceramide. hFA-ceramides in epidermis support permeability barrier function. hFA-ceramides are synthesized by fatty acid 2-hydroxylase (FA2H). Mutations of FA2H causes neurological disorders including, leukodystrophy and spastic paraparesis in humans. The mechanism of action for an antitumor drug, PM02734 involves hFA-ceramides in the cell membrane.
Packaging
5 mL Amber Glass Screw Cap Vial (860815P-25mg)
5 mL Amber Glass Screw Cap Vial (860815P-5mg)
Legal Information
Avanti Research is a trademark of Avanti Polar Lipids, LLC
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Levels of SCS7/FA2H-mediated fatty acid 2-hydroxylation determine the sensitivity of cells to antitumor PM02734
Herrero AB, et al.
Cancer research, 68(23), 9779-9787 (2008)
Fatty acid 2-Hydroxylation in mammalian sphingolipid biology
Hama H, et al.
Biochimica et Biophysica Acta - Molecular and Cell Biology of Lipids, 1801(4), 405-414 (2010)
Ana B Herrero et al.
Cancer research, 68(23), 9779-9787 (2008-12-03)
PM02734 is a novel synthetic antitumor drug that is currently in phase I clinical trials. To gain some insight into its mode of action, we used the yeast Saccharomyces cerevisiae as a model system. Treatment of S. cerevisiae with PM02734
Nathan L Alderson et al.
Journal of lipid research, 50(6), 1203-1208 (2009-01-28)
Sphingolipids are ubiquitous components of eukaryotic cells that regulate various cellular functions. In many cell types, a fraction of sphingolipids contain 2-hydroxy fatty acids, produced by fatty acid 2-hydroxylase (FA2H), as the N-acyl chain of ceramide [hydroxyl fatty acid (hFA)-sphingolipids].
Hiroko Hama
Biochimica et biophysica acta, 1801(4), 405-414 (2009-12-23)
2-Hydroxy fatty acids (hFA) are important components of a subset of mammalian sphingolipids. The presence of hFA in sphingolipids is best described in the nervous system, epidermis, and kidney. However, the literature also indicates that various hFA-sphingolipids are present in
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service