W381829
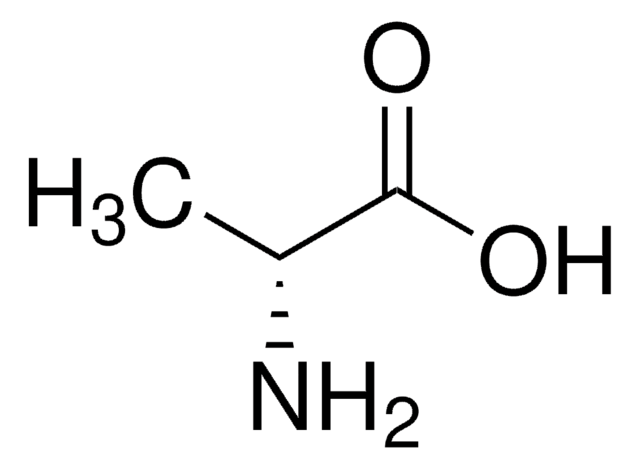
L-Alanine
≥99%
Synonym(s):
(S)-2-Aminopropionic acid, L-α-Aminopropionic acid
About This Item
Recommended Products
biological source
synthetic
Quality Level
Assay
≥99%
form
chunks
crystalline powder
powder
optical activity
[α]20/D +13.5 to +15.5°, c = 10 in 6 M HCl
solubility
H2O: soluble 89.1 g/L at 20 °C (completely)
application(s)
flavors and fragrances
Documentation
see Safety & Documentation for available documents
food allergen
no known allergens
Organoleptic
sweet
SMILES string
C[C@H](N)C(O)=O
InChI
1S/C3H7NO2/c1-2(4)3(5)6/h2H,4H2,1H3,(H,5,6)/t2-/m0/s1
InChI key
QNAYBMKLOCPYGJ-REOHCLBHSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
- Exposure to synthesized tribromobisphenol A and critical effects: Metabolic pathways, disease signature, and benchmark dose derivation.: This study provides insight into the metabolic pathways and disease signatures associated with exposure to synthesized tribromobisphenol A, emphasizing the critical role of L-alanine in mediating these effects (Kuang et al., 2024).
- Prenylated indole diketopiperazine alkaloids as phosphatase inhibitors from the marine-derived fungus Talaromyces purpureogenus.: Identifies new prenylated indole diketopiperazine alkaloids from Talaromyces purpureogenus that act as potent phosphatase inhibitors, with L-alanine being key to their structure and bioactivity, offering potential for drug development (Liang et al., 2024).
Disclaimer
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service