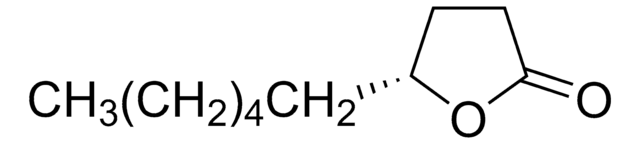W361305
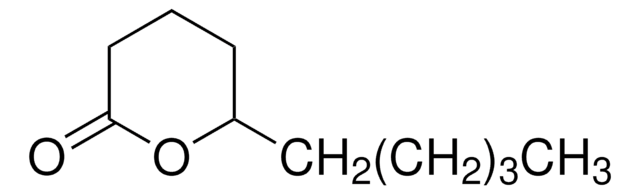
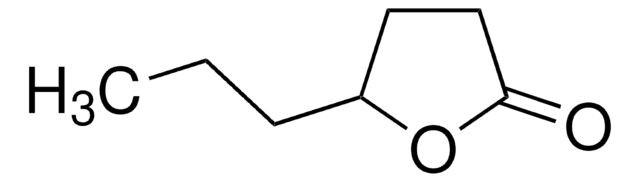
ε-Decalactone
≥99%
Synonym(s):
7-Butyl-2-oxepanone
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C10H18O2
CAS Number:
Molecular Weight:
170.25
FEMA Number:
3613
EC Number:
MDL number:
UNSPSC Code:
12164502
PubChem Substance ID:
Flavis number:
10.029
NACRES:
NA.21
Recommended Products
biological source
synthetic
Quality Level
Agency
meets purity specifications of JECFA
Assay
≥99%
refractive index
n20/D 1.461 (lit.)
bp
117 °C/3 mmHg (lit.)
density
0.976 g/mL at 25 °C (lit.)
application(s)
flavors and fragrances
Organoleptic
coconut; creamy; sweet
SMILES string
CCCCC1CCCCC(=O)O1
InChI
1S/C10H18O2/c1-2-3-6-9-7-4-5-8-10(11)12-9/h9H,2-8H2,1H3
InChI key
YKVIWISPFDZYOW-UHFFFAOYSA-N
Related Categories
Disclaimer
For R&D or non-EU Food use. Not for retail sale.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 2
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
M Furukawa et al.
Biochemical and biophysical research communications, 199(1), 41-45 (1994-02-28)
Lipase from Pseudomonas cepacia was modified with 2,4-bis[O-methoxypoly(ethylene glycol)]-6-chloro-s-triazine, activated PEG2, to form PEG-lipase. The PEG-lipase is soluble and active in organic solvents. It catalyzes alcoholysis of racemic epsilon-decalactone with ethanol in 1,1,1-trichloroethane to form (R)-hydroxydecanoic acid ethyl ester. No
K Juni et al.
Critical reviews in therapeutic drug carrier systems, 3(3), 209-232 (1987-01-01)
Poly(hydroxy acids) so far have been examined for use in drug delivery in limited number, while the advantageous use of the polymers has been recognized due to their biodegradability and biocompatibility. Homo- and copolymers of lactic acid and glycolic acid
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service