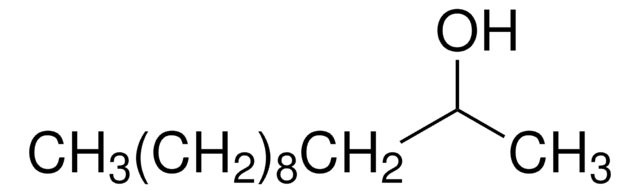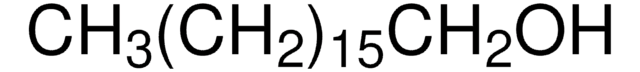U1001
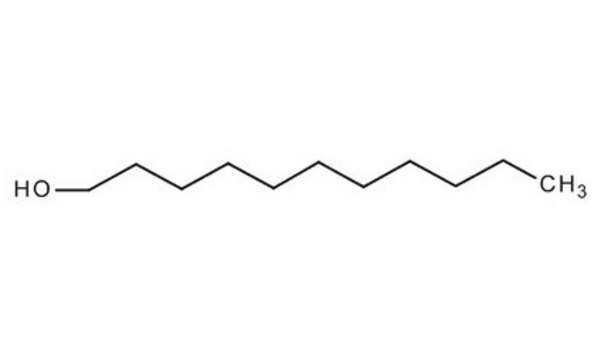
1-Undecanol
97%
Synonym(s):
Alcohol C11, Undecyl alcohol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3(CH2)10OH
CAS Number:
Molecular Weight:
172.31
Beilstein:
1698334
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
refractive index
n20/D 1.44 (lit.)
bp
146 °C/30 mmHg (lit.)
mp
11 °C (lit.)
density
0.83 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
CCCCCCCCCCCO
InChI
1S/C11H24O/c1-2-3-4-5-6-7-8-9-10-11-12/h12H,2-11H2,1H3
InChI key
KJIOQYGWTQBHNH-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
1-Undecanol can be used as a precursor in the synthesis of undecanal by chemoselective oxidation using a fluorous derivative of TEMPO (2,2,6,6-tetramethylpiperidine-1-oxyl) radical as a catalyst.
It can be also used as a solvent in homogeneous liquid-liquid microextraction method.
It can be also used as a solvent in homogeneous liquid-liquid microextraction method.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Acute 1 - Aquatic Chronic 2 - Eye Irrit. 2
Storage Class Code
10 - Combustible liquids
WGK
WGK 2
Flash Point(F)
226.4 °F - closed cup
Flash Point(C)
108 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Determination of polycyclic aromatic hydrocarbons in soil samples using flotation-assisted homogeneous liquid-liquid microextraction
Hosseini MH, et al.
Journal of Chromatography A, 1265(22), 52-56 (2012)
Synthesis and catalytic activity of a fluorous-tagged TEMPO radical
Pozzi G, et al.
Tetrahedron Letters, 45(22), 4249-4251 (2004)
Junheon Kim et al.
Scientific reports, 6, 29300-29300 (2016-07-13)
2-(1-Undecyloxy)-1-ethanol, monochamol, is a male-produced aggregation pheromone of the Monochamus species, which are efficient vectors of the pine wood nematode (PWN), Bursaphelenchus xylophilus, which cause devastating damage to pines worldwide. The nematicidal activity of synthetic monochamol and its homologues (ROEtOH:
Shivalika Tanwar et al.
Carbohydrate polymers, 217, 26-34 (2019-05-14)
Cyclodextrins are supramolecules widely used to help solubilization of hydrophobic molecules via host: guest complexation. To prepare the complexes, co-solvents like alcohols are mandatory and play an important role in the complexation process. In particular, the length of the aliphatic
Fateme Mirrahimi et al.
Journal of AOAC International, 97(3), 933-937 (2014-07-24)
A sensitive and selective method for the determination of low levels of rhodium (Rh) in environmental samples is needed. In the proposed method, an extracting solvent with a lower toxicity and density than the other solvents typically used in dispersive
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service