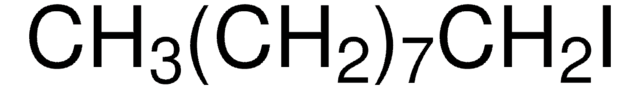T76805
2,3,3-Trimethylindolenine
98%
Synonym(s):
2,3,3-Trimethyl-3H-indole
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C11H13N
CAS Number:
Molecular Weight:
159.23
Beilstein:
119740
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
refractive index
n20/D 1.549 (lit.)
bp
228-229 °C/744 mmHg (lit.)
density
0.992 g/mL at 25 °C (lit.)
SMILES string
CC1=Nc2ccccc2C1(C)C
InChI
1S/C11H13N/c1-8-11(2,3)9-6-4-5-7-10(9)12-8/h4-7H,1-3H3
InChI key
FLHJIAFUWHPJRT-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
2,3,3-Trimethylindolenine is an indolenine compound used as a reactant in organic synthesis reactions.
Application
- Squarylium-based chromogenic anion sensors.: This research explores the synthesis and application of squarylium-based sensors, which includes 2,3,3-Trimethylindolenine as a crucial component. The study focuses on the effectiveness of these sensors in detecting various anions through chromogenic changes, highlighting the compound′s role in developing advanced chemical sensing technologies (Lee EM et al., 2012).
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Flash Point(F)
199.4 °F - closed cup
Flash Point(C)
93 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Chanuk Jeong et al.
Journal of controlled release : official journal of the Controlled Release Society, 329, 50-62 (2020-12-02)
Near-infrared (NIR)-induced dye-based theranostic drug delivery carriers are used for critical image-guided chemo-photothermal cancer therapy. However, most carriers fail to deliver sufficient heat and fluorescence efficiently due to direct π-π stacking of the aromatic rings of the NIR dye and
Juewei Ning et al.
Molecular medicine reports, 15(6), 3761-3766 (2017-04-26)
The future of personalized cancer treatments relies on the development of functional agents that have tumor-targeted anticancer activities and can be detected in tumors using imaging. However, application of these functional agents in the clinic has been limited due to
Hang Zhang et al.
Nature communications, 10(1), 3267-3267 (2019-07-25)
Living systems have inspired research on non-biological dynamic materials and systems chemistry to mimic specific complex biological functions. Upon pursuing ever more complex life-inspired non-biological systems, mimicking even the most elementary aspects of learning is a grand challenge. We demonstrate
Antonio Setaro et al.
Nature communications, 8, 14281-14281 (2017-01-31)
Covalent functionalization tailors carbon nanotubes for a wide range of applications in varying environments. Its strength and stability of attachment come at the price of degrading the carbon nanotubes sp
Ilkoo Noh et al.
Advanced science (Weinheim, Baden-Wurttemberg, Germany), 5(3), 1700481-1700481 (2018-03-30)
A noninvasive and selective therapy, photodynamic therapy (PDT) is widely researched in clinical fields; however, the lower efficiency of PDT can induce unexpected side effects. Mitochondria are extensively researched as target sites to maximize PDT effects because they play crucial
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![1,1,2-Trimethylbenz[e]indole ≥98.0% (HPLC)](/deepweb/assets/sigmaaldrich/product/structures/296/735/4c0b92e3-1a5f-4c32-8b5b-0b0997c15df4/640/4c0b92e3-1a5f-4c32-8b5b-0b0997c15df4.png)
![1′,3′-Dihydro-1′,3′,3′-trimethyl-6-nitrospiro[2H-1-benzopyran-2,2′-(2H)-indole] 98%](/deepweb/assets/sigmaaldrich/product/structures/503/745/147ecd2c-44b9-46e9-a8c9-bff9a2577218/640/147ecd2c-44b9-46e9-a8c9-bff9a2577218.png)
![N-[5-(Phenylamino)-2,4-pentadienylidene]aniline monohydrochloride 98%](/deepweb/assets/sigmaaldrich/product/structures/365/246/6dec3589-6c56-4b0a-a3ae-e8d5fbbd3f05/640/6dec3589-6c56-4b0a-a3ae-e8d5fbbd3f05.png)