T7394
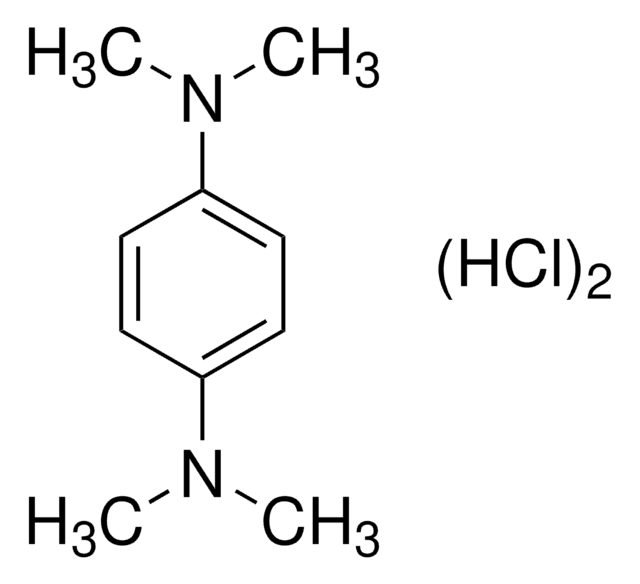
N,N,N′,N′-Tetramethyl-p-phenylenediamine
99%, powder
Synonym(s):
TMPD, TMPDA, TMPPD, Wurster’s reagent
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
C6H4[N(CH3)2]2
Molecular Weight:
164.25
Beilstein:
1564025
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
flakes
powder
color
off-white to brown
bp
260 °C (lit.)
mp
49-51 °C (lit.)
SMILES string
CN(C)c1ccc(cc1)N(C)C
InChI
1S/C10H16N2/c1-11(2)9-5-7-10(8-6-9)12(3)4/h5-8H,1-4H3
InChI key
CJAOGUFAAWZWNI-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
N,N,N′,N′-Tetramethyl-p-phenylenediamine (TMPDA) is a redox reagent with low ionization potential widely used as an electron donor for photosystem I. It also acts as an electron acceptor in photosystem II.
Application
N,N,N′,N′-Tetramethyl-p-phenylenediamine (TMPDA) can be used:
- In the flow injection analysis of benzoyl peroxide.
- To study photoinduced electron transfer to halogenated solvents.
Caution
May darken in storage.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
230.0 °F - closed cup
Flash Point(C)
110 °C - closed cup
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Ultrafast photoinduced electron transfer from N, N, N′ , N′ -tetramethyl-p-phenylenediamine and N, N, N′ , N′ -tetramethylbenzidine to dichloromethane
Boilet L, et al.
Journal of Photochemistry and Photobiology A: Chemistry, 163(3), 529-536 (2004)
Flow injection analysis of benzoyl peroxide using N, N, N, N-tetramethyl-p-phenylenediamine (TMPDA) and surfactants
Pharr DY & Tomsyck JA
Analytical Letters, 42(5), 821-832 (2009)
Interaction of N, N, N′ ,N′ -tetramethyl-p-phenylenediamine with photosystem II as revealed by thermoluminescence: reduction of the higher oxidation states of the Mn cluster and displacement of plastoquinone from the QB niche
Gauthier A, et al.
Biochimica et Biophysica Acta, 1757(11), 1547-1556 (2006)
P P Bawol et al.
Physical chemistry chemical physics : PCCP, 20(33), 21447-21456 (2018-08-09)
The reversibility of current Li-O2 batteries suffers from high charging overpotentials. To address this problem, the use of redox mediators has been proposed, which are supposed to improve the sluggish reaction kinetics of the oxygen evolution reaction via a solution
Toshiaki Miura
Chemico-biological interactions, 236, 67-73 (2015-04-30)
To investigate the mechanisms of cardiotoxicity induced by adriamycin (ADM), the enzymatic activities of ADM-Fe(3+), including the peroxidase and lipoxygenase (LOX) activity, and participation of active oxygen species in the damage to biological components were examined. ADM-Fe(3+), but not ADM
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service