P21404
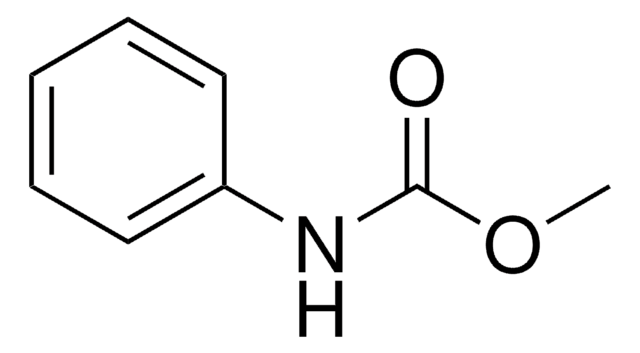
Phenyl carbamate
97%
Synonym(s):
O-Phenyl carbamate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
H2NCO2C6H5
CAS Number:
Molecular Weight:
137.14
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
powder
mp
149-152 °C (lit.)
SMILES string
NC(=O)Oc1ccccc1
InChI
1S/C7H7NO2/c8-7(9)10-6-4-2-1-3-5-6/h1-5H,(H2,8,9)
InChI key
BSCCSDNZEIHXOK-UHFFFAOYSA-N
Related Categories
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Amani Atayat et al.
Journal of separation science, 42(18), 3023-3032 (2019-06-30)
The aim of this work was to develop an efficient method for the selective extraction and analysis of fenoxycarb, a carbamate pesticide, in mussel samples using a molecularly imprinted solid-phase extraction device. The optimization of molecularly imprinted polymer synthesis was
Chin Fen Teo et al.
Glycobiology, 26(11), 1198-1208 (2016-04-14)
Previous studies utilizing PUGNAc, the most widely used β-N-acetylglucosaminidase (OGA) inhibitor to increase global O-N-acetylglucosamine (GlcNAc) levels, have reported a variety of effects including insulin resistance as a direct result of elevated O-GlcNAc levels. The notion of OGA inhibition causing
S A Adediran et al.
Archives of biochemistry and biophysics, 618, 23-31 (2017-01-29)
The best-studied amidase signature (AS) enzyme is probably fatty acid amide hydrolase (FAAH). Closely related to FAAH is mandelamide hydrolase (MAH), whose substrate specificity and mechanism of catalysis are described in this paper. First, we developed a convenient chromogenic substrate
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service