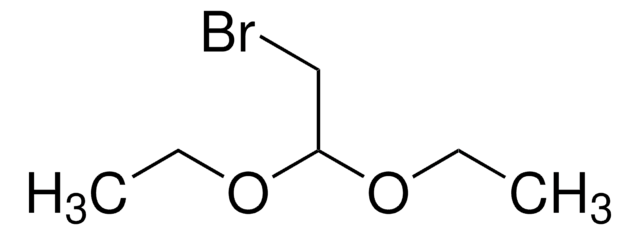
M28006
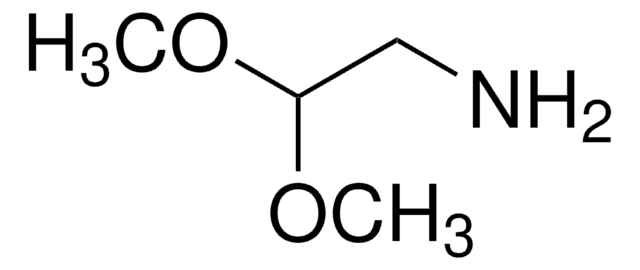
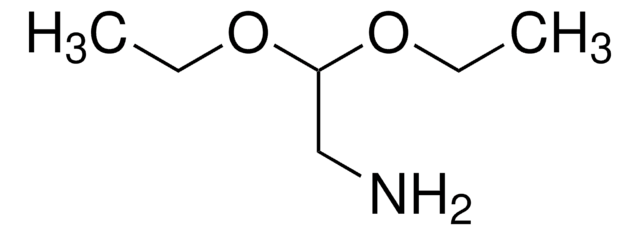
(Methylamino)acetaldehyde dimethyl acetal
97%
Synonym(s):
1,1-Dimethoxy-2-methylaminoethane, 2,2-Dimethoxy-N-methylethylamine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3NHCH2CH(OCH3)2
CAS Number:
Molecular Weight:
119.16
Beilstein:
605322
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
liquid
refractive index
n20/D 1.414 (lit.)
bp
140 °C (lit.)
density
0.928 g/mL at 25 °C (lit.)
SMILES string
CNCC(OC)OC
InChI
1S/C5H13NO2/c1-6-4-5(7-2)8-3/h5-6H,4H2,1-3H3
InChI key
HUMIEJNVCICTPJ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
(Methylamino)acetaldehyde dimethyl acetal can be used as a reactant to synthesize:
- Aza[3.3.2] cyclazines by reacting with 5-methyloxazolo[3,2-a]pyridinium salts via synthesis of functionalized 5-aminoindolizines intermediates.
- N-(2,2-Dimethoxyethyl)-N-methyl-3,4-dimethoxyphenylglycine by Petasis reaction with glyoxylic acid and 3,4-dimethoxyphenylboronic acid.
- Substituted imidazoles via copper-catalyzed reaction with various nitriles.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
84.2 °F
Flash Point(C)
29 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A concise synthesis of tetrahydroisoquinoline-1-carboxylic acids using a Petasis reaction and Pomeranz-Fritsch-Bobbitt cyclization sequence
Chrzanowska M, et al.
Tetrahedron, 68(14), 3092-3097 (2012)
Expedient synthesis of substituted imidazoles from nitriles
Frutos RP, et al.
Tetrahedron Letters, 46(48), 8369-8372 (2005)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service