M1641
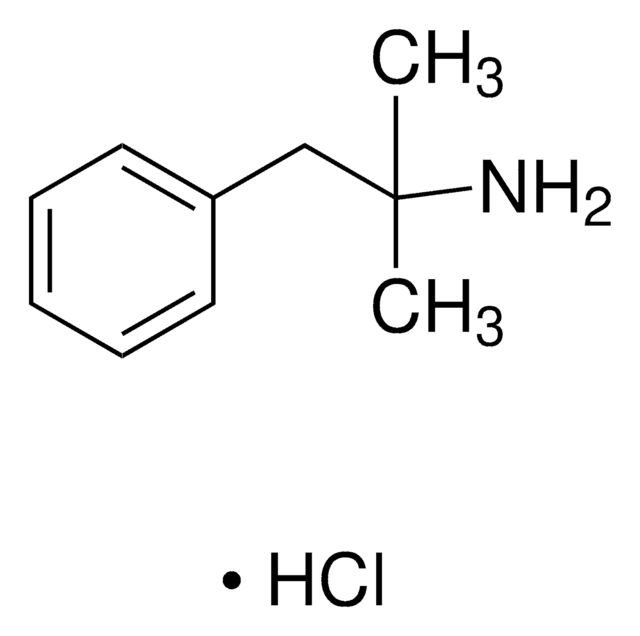
Methoxyphenamine hydrochloride
≥99%
Synonym(s):
2-Methoxy-N,α-dimethylbenzeneethanamine hydrochloride, NSC 65644
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C11H17NO · HCl
CAS Number:
Molecular Weight:
215.72
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥99%
drug control
regulated under CDSA - not available from Sigma-Aldrich Canada
SMILES string
Cl.CNC(C)Cc1ccccc1OC
InChI
1S/C11H17NO.ClH/c1-9(12-2)8-10-6-4-5-7-11(10)13-3;/h4-7,9,12H,8H2,1-3H3;1H
InChI key
FGSJNNQVSUVTPW-UHFFFAOYSA-N
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Hajime Miyaguchi et al.
Journal of chromatography. A, 1129(1), 105-110 (2006-07-29)
A microchip-based liquid-liquid extraction for the gas chromatography analysis of urine for amphetamine-type stimulants has been developed. Partially modified microchannels with the capillarity restricted modification (CARM) method were employed for stabilizing the interface consisting of 1-chlorobutane and alkalized urine. Reliability
S Geertsen et al.
Xenobiotica; the fate of foreign compounds in biological systems, 25(9), 895-906 (1995-09-01)
1. Control and P4502D6-transfected human B-lymphoblastoid cell lines (cHol and h2D6v2 respectively) were used to study 2D6-mediated metabolism of methoxyphenamine (MPA) and 2-methoxyamphetamine (2MA). The main metabolites were products of O-dealkylation and aromatic hydroxylation at the 5-position. In addition, N-desmethyl-methoxyphenamine
N R Srinivas et al.
Journal of chromatography, 487(1), 61-72 (1989-01-27)
Sensitive and enantioselective gas chromatographic assays have been developed and applied to the quantitation in human urine of the enantiomers of methoxyphenamine and its three primary oxidative metabolites, namely, N-desmethylmethoxyphenamine, O-desmethylmethoxyphenamine and 5-hydroxymethoxyphenamine. The separation of the various analytes was
G Muralidharan et al.
Xenobiotica; the fate of foreign compounds in biological systems, 21(11), 1441-1450 (1991-11-01)
1. Lewis rats (n = 7 or 8) were dosed with methoxyphenamine with and without prior administration of various doses of either quinine or its diastereomer quinidine. Methoxyphenamine and its N-desmethyl, O-desmethyl and aromatic 5-hydroxy metabolites were quantified in 0-24
Y Nakahara et al.
Forensic science international, 63(1-3), 109-119 (1993-12-01)
The excretion of methoxyphenamine (MOP) and methamphetamine (MA) into beards has been studied. Six healthy male subjects orally took 50 mg of MOP at a single dose and 7 doses for a successive 7 days. Their beard hairs were collected
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service