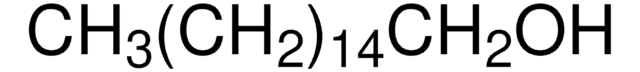H6800
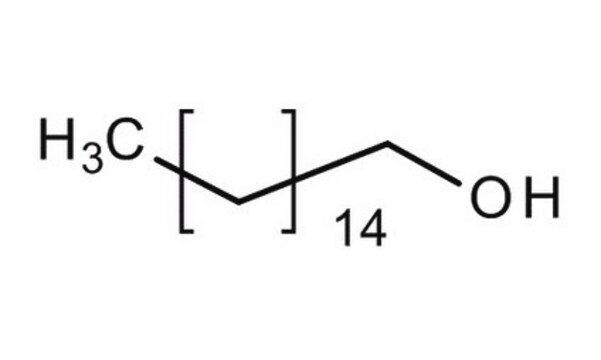
1-Hexadecanol
95%
Synonym(s):
Cetyl alcohol, Palmityl alcohol
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
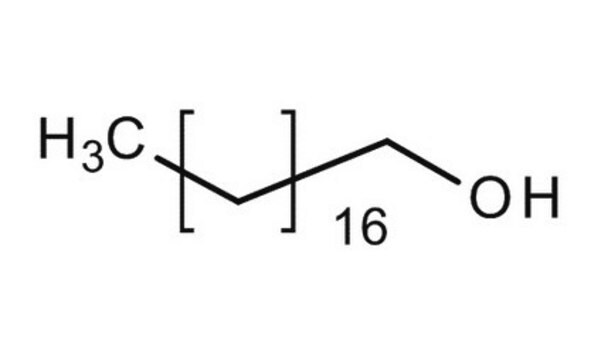
CH3(CH2)15OH
CAS Number:
Molecular Weight:
242.44
Beilstein:
1748475
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor density
8.34 (vs air)
Quality Level
vapor pressure
<0.01 mmHg ( 43 °C)
Assay
95%
form
solid
autoignition temp.
483 °F
expl. lim.
8 %
bp
179-181 °C/10 mmHg (lit.)
mp
48-50 °C (lit.)
density
0.818 g/mL at 25 °C (lit.)
SMILES string
CCCCCCCCCCCCCCCCO
InChI
1S/C16H34O/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17/h17H,2-16H2,1H3
InChI key
BXWNKGSJHAJOGX-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- 1-Hexadecanol can be used as a reactant in the synthesis of self-assembled m-diethynylbenzene macrocycles (DBMs), water-soluble pillar[6]arene for controllable drug release and poly(ethylene glycol)-based polymer for intracellular delivery of anticancer drugs.
- It is used in building the template for sol-gel synthesis of mesoporous silicates.
- It can be employed in the synthesis of anionic gemini surfactants which also exhibit antibacterial and antifungal activities.
- High-chain fatty acid esters of 1-hexadecanol can be used as phase change materials (PCM) for thermal energy storage.
Storage Class Code
11 - Combustible Solids
WGK
nwg
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Multistimuli-responsive supramolecular vesicles based on water-soluble pillar [6] arene and SAINT complexation for controllable drug release.
Cao Y, et al.
Journal of the American Chemical Society, 136(30), 10762-10769 (2014)
A condensable amphiphile with a cleavable tail as a ?lizard? template for the sol? gel synthesis of functionalized mesoporous silica.
Zhang Q, et al.
Journal of the American Chemical Society, 126(4), 988-989 (2004)
Cellular uptake, intracellular trafficking, and antitumor efficacy of doxorubicin-loaded reduction-sensitive micelles.
Cui C, et al.
Biomaterials, 34(15), 3858-3869 (2013)
Characterization, surface properties and biological activity of some synthesized anionic surfactants.
Negm N A and Tawfik S M
Journal of Industrial and Engineering Chemistry, 20(6), 4463-4472 (2014)
m-Diethynylbenzene macrocycles: syntheses and self-association behavior in solution.
Tobe Y, et al.
Journal of the American Chemical Society, 124(19), 5350-5364 (2002)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service