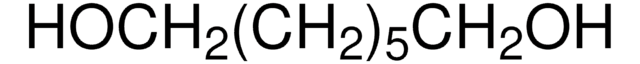H11807
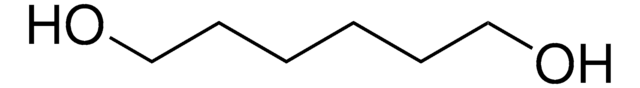
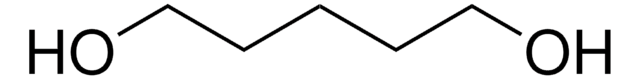
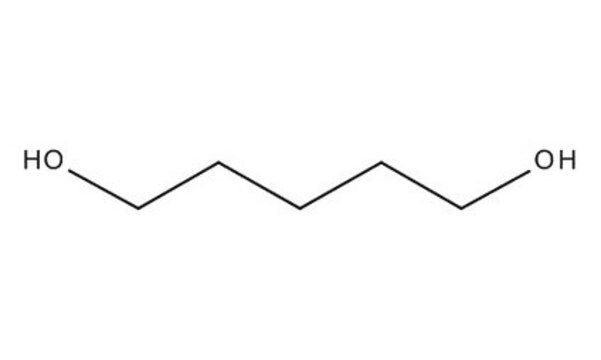
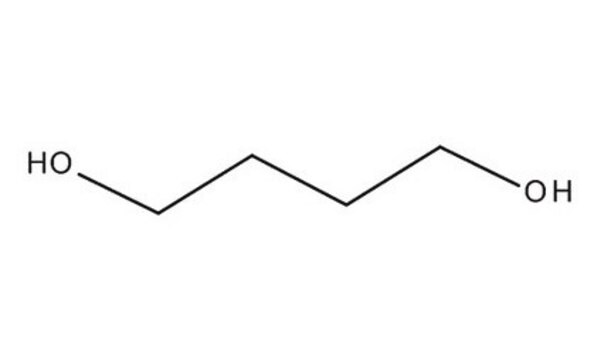
1,6-Hexanediol
97%
Synonym(s):
Hexamethylene glycol
Sign Into View Organizational & Contract Pricing
All Photos(4)
About This Item
Linear Formula:
HO(CH2)6OH
CAS Number:
Molecular Weight:
118.17
Beilstein:
1633461
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor pressure
0.53 mmHg ( 20 °C)
Quality Level
Assay
97%
autoignition temp.
608 °F
expl. lim.
16 %
bp
250 °C (lit.)
mp
38-42 °C (lit.)
SMILES string
OCCCCCCO
InChI
1S/C6H14O2/c7-5-3-1-2-4-6-8/h7-8H,1-6H2
InChI key
XXMIOPMDWAUFGU-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
1,6-Hexanediol is generally used to introduce C6-spacer in molecular substrates. It is also widely used in the synthesis of various polyesters.
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
215.6 °F - closed cup
Flash Point(C)
102 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Melt-phase synthesis and properties of triptycene-containing copolyesters.
Liu, Yanchun et al.
Macromolecules, 44(11), 4049-4056 (2011)
Liquid crystalline phthalocyanine-fullerene dyads.
Ince, Mine et al.
Journal of Materials Chemistry, 21(5), 1531-1536 (2011)
A series of furan-aromatic polyesters synthesized via direct esterification method based on renewable resources.
Jiang, Min et al.
Journal of Polymer Science Part A: Polymer Chemistry, 50(5), 1026-1036 (2012)
Kifah Nasr et al.
Polymers, 12(9) (2020-08-28)
Among the various catalysts that can be used for polycondensation reactions, enzymes have been gaining interest for three decades, offering a green and eco-friendly platform towards the sustainable design of renewable polyesters. However, limitations imposed by their delicate nature, render
H D Durham et al.
Muscle & nerve, 11(2), 160-165 (1988-02-01)
We reported previously that 2,5-hexanedione (2,5-HD), the neurotoxic metabolite of methyl-n-butylketone (MnBK) and n-hexane, induced aggregation of intermediate filaments of the vimentin type in cultured fibroblasts. To determine if these findings have relevance to the mechanism by which these hexacarbons
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service