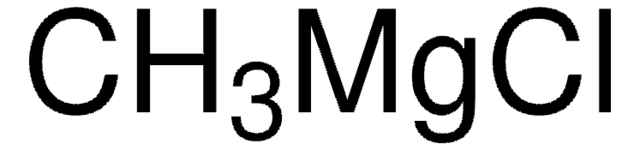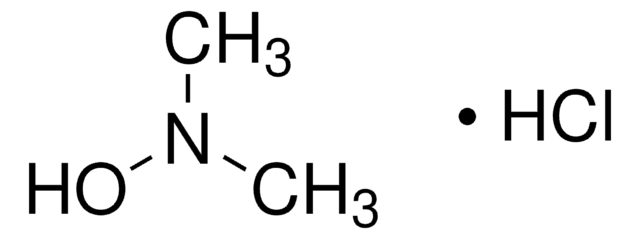D163708
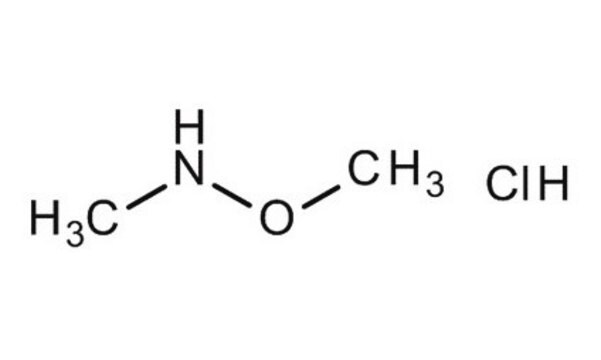
N,O-Dimethylhydroxylamine hydrochloride
98%
Synonym(s):
N,O-Dimethylhydroxylamine HCl, N-Methoxymethylamine hydrochloride, Methoxymethylamine hydrochloride
Sign Into View Organizational & Contract Pricing
All Photos(5)
About This Item
Linear Formula:
CH3ONHCH3 · HCl
CAS Number:
Molecular Weight:
97.54
Beilstein:
3650353
EC Number:
MDL number:
UNSPSC Code:
12352107
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
crystals
mp
112-115 °C
SMILES string
Cl[H].CNOC
InChI
1S/C2H7NO.ClH/c1-3-4-2;/h3H,1-2H3;1H
InChI key
USZLCYNVCCDPLQ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Reagent for the preparation of Weinreb amides recently used in the synthesis of 2-acyloxazoles from 2-oxazolemagnesium chloride.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Daniel J Pippel et al.
The Journal of organic chemistry, 72(15), 5828-5831 (2007-06-26)
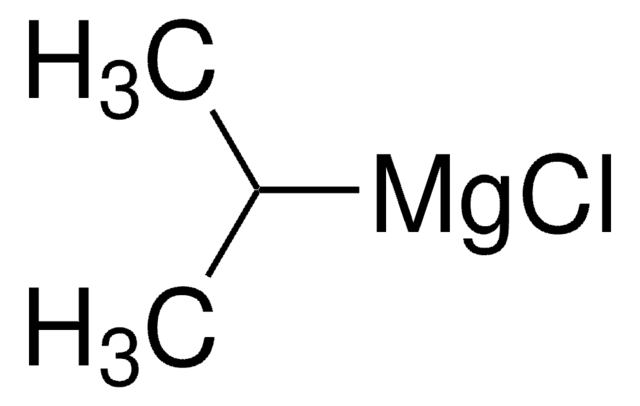
Treatment of oxazole or 5-aryl oxazoles with i-PrMgCl smoothly generates the corresponding 2-Grignard reagents, which react with Weinreb amides to provide exclusively 2-acyl oxazole products.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service