D144002
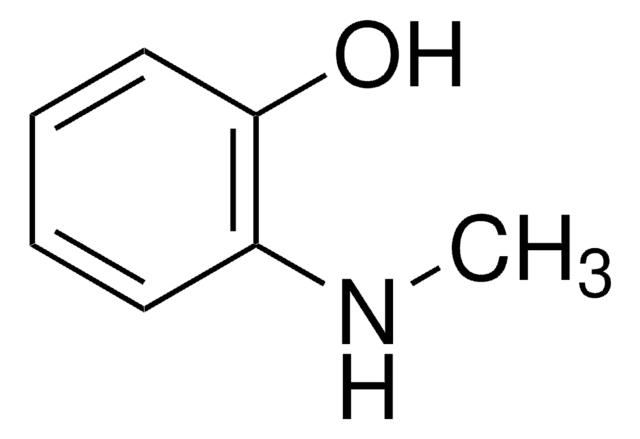
3-(Dimethylamino)phenol
97%
Synonym(s):
N,N-Dimethyl-3-aminophenol
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
(CH3)2NC6H4OH
CAS Number:
Molecular Weight:
137.18
Beilstein:
774631
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
crystals
mp
82-84 °C (lit.)
SMILES string
CN(C)c1cccc(O)c1
InChI
1S/C8H11NO/c1-9(2)7-4-3-5-8(10)6-7/h3-6,10H,1-2H3
InChI key
MESJRHHDBDCQTH-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
298.4 °F - closed cup
Flash Point(C)
148 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Anežka Tichá et al.
The Journal of biological chemistry, 292(7), 2703-2713 (2017-01-11)
Rhomboid proteases are increasingly being explored as potential drug targets, but their potent and specific inhibitors are not available, and strategies for inhibitor development are hampered by the lack of widely usable and easily modifiable
Bozena Bukowska et al.
Toxicology in vitro : an international journal published in association with BIBRA, 21(8), 1574-1580 (2007-07-17)
3-(Dimethylamino)phenol (3-DMAP) exists in the environment as a transformation product of ureic herbicides and may also be considered as a derivative of phenoxyherbicides. In this study, the activity of glutathione peroxidase, catalase and superoxide dismutase, as well as the level
Avi Weissberg et al.
Journal of chromatography. A, 1512, 71-77 (2017-07-18)
A methodology for sensitive determination of sarin (GB), soman (GD) and cyclosarin (GF) chemical warfare agents in aqueous media was developed. The method incorporates direct derivatization with 2-[(dimethylamino)methyl]phenol (2-DMAMP), a commercially available, water-soluble reagent, followed by LC-ESI-MS/MS analysis in the
Cynthia D Selassie et al.
Journal of medicinal chemistry, 48(23), 7234-7242 (2005-11-11)
In this comprehensive study on the caspase-mediated apoptosis-inducing effect of 51 substituted phenols in a murine leukemia cell line (L1210), we determined the concentrations needed to induce caspase activity by 50% (I50) and utilized these data to develop the following
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service