D107808
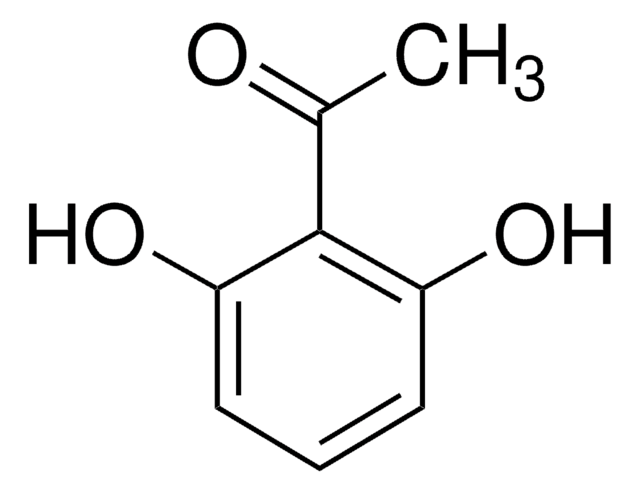
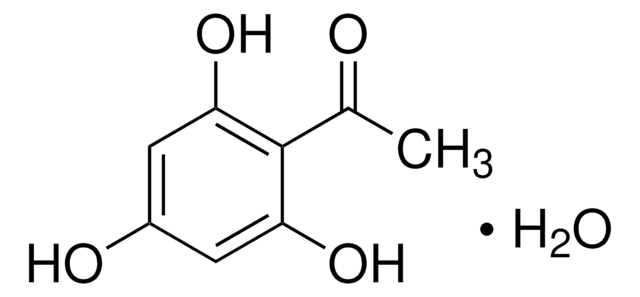
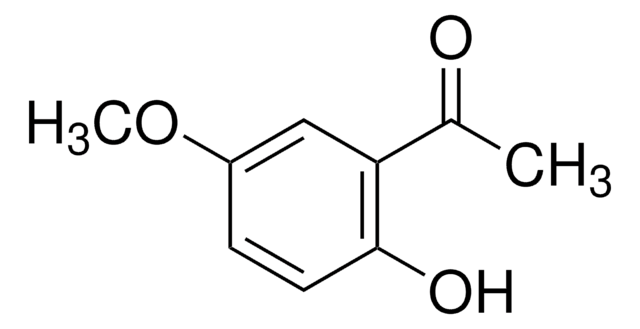
2′,6′-Dihydroxyacetophenone
97%
Synonym(s):
2-Acetyl-1,3-dihydroxybenzene, 2-Acetylresorcinol, DHAP
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(HO)2C6H3COCH3
CAS Number:
Molecular Weight:
152.15
Beilstein:
1366061
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
powder
mp
156-158 °C (lit.)
SMILES string
CC(=O)c1c(O)cccc1O
InChI
1S/C8H8O3/c1-5(9)8-6(10)3-2-4-7(8)11/h2-4,10-11H,1H3
InChI key
YPTJKHVBDCRKNF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
MALDI mass spectrometry in the solution of some forensic problems.
R Seraglia et al.
Forensic science international, 146 Suppl, S83-S85 (2005-01-11)
Gheorghita Zbancioc et al.
Ultrasonics sonochemistry, 19(3), 399-403 (2011-09-13)
A new, selective, straightforward and general method for preparation of highly functionalized coronands or spiro derivatives bearing 1,2-dihydroxyacetophenone unit, under conventional conditions and ultrasonic irradiation, is reported. The reaction setup involves only one step, acylation of an α-chloro-3,4-dihydroxyacetophenone with phthaloyl
J J Gorman et al.
Rapid communications in mass spectrometry : RCM, 10(5), 529-536 (1996-01-01)
Several peptides were shown to undergo fragmentation during matrix-assisted laser desorption/ionization time-of-flight mass spectrometry to a degree which complicated their analysis using alpha-cyano-4-hydroxycinnamic acid (CHCA) as a matrix, even at threshold laser irradiance. These peptides included synthetic peptides, peptides isolated
Junjie Hou et al.
Journal of biomedicine & biotechnology, 2010, 759690-759690 (2010-03-27)
Selecting an appropriate matrix solution is one of the most effective means of increasing the ionization efficiency of phosphopeptides in matrix-assisted laser-desorption/ionization time-of-flight mass spectrometry (MALDI-TOF-MS). In this study, we systematically assessed matrix combinations of 2, 6-dihydroxyacetophenone (DHAP) and diammonium
Chong-Feng Xu et al.
Analytical chemistry, 79(5), 2007-2014 (2007-01-26)
We have developed a new strategy to enrich and fractionate phosphopeptides from peptide mixtures based on the difference in their isoelectric points (pIs) after methyl esterification. After isoelectric focusing (IEF) of a methylated tryptic digest of a mixture of alpha-S-casein
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service