All Photos(2)
About This Item
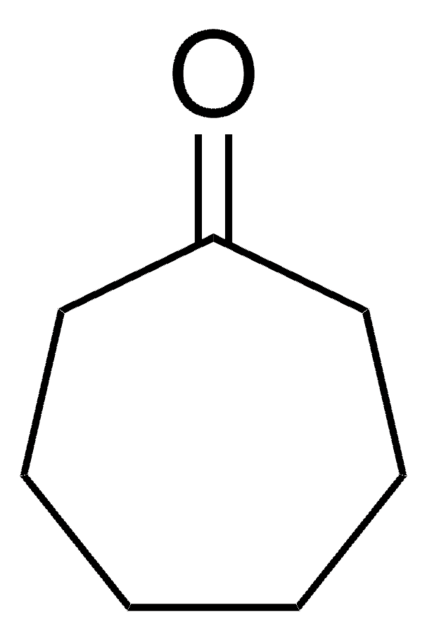
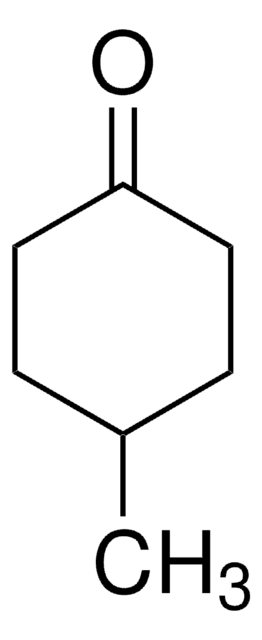
Linear Formula:
C8H14(=O)
CAS Number:
Molecular Weight:
126.20
Beilstein:
1280738
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
crystals
bp
195-197 °C (lit.)
mp
32-41 °C (lit.)
density
0.958 g/mL at 25 °C (lit.)
SMILES string
O=C1CCCCCCC1
InChI
1S/C8H14O/c9-8-6-4-2-1-3-5-7-8/h1-7H2
InChI key
IIRFCWANHMSDCG-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
165.2 °F
Flash Point(C)
74 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Vishwakarma Singh et al.
The Journal of organic chemistry, 70(3), 973-981 (2005-01-29)
A new and efficient synthesis of a variety of highly embellished bicyclooctenones having an endo-vinyl moiety and their sigmatropic shifts in ground and excited states leading to a stereoselective route to substituted cis-decalins and diquinane frameworks have been described. Functionalized
M E Krafft et al.
The Journal of organic chemistry, 66(22), 7443-7448 (2001-10-30)
The total synthesis of asteriscanolide (1) has been achieved by taking advantage on an intermolecular Pauson-Khand cycloaddition and a ring-closing metathesis as key bond-forming transformations. The approach incorporates the cyclooctane stereogenic center prior to ring formation. Interestingly, the ring-closing metathesis
K Yamada et al.
Chemical & pharmaceutical bulletin, 45(12), 1898-1905 (1998-01-20)
Construction of the AB-ring system of the taxane framework via an A-ring annulation strategy was demonstrated by base-mediated intramolecular aldol reaction of (Z)-2,2-dimethyl-3-(1-methyl-2-oxopropylidene)cyclooctanone, affording the title compound, 1-hydroxy-8,11,11-trimethylbicyclo[5.3.1]undec-7-en-9-one. A cyclization precursor, the tetra-substituted (Z)-alkene, was prepared from the corresponding cyclooctanone
F E Harvey et al.
Brain research bulletin, 13(4), 541-547 (1984-10-01)
Female mice were reared in observation incubators from day 1 of life for three weeks. During that time they were continuously exposed to the odors of either cyclooctanone, adult male mouse urine or distilled water. The growth rate was temporarily
K Yamada et al.
Chemical & pharmaceutical bulletin, 45(12), 2113-2115 (1998-01-20)
Stereoselective syntheses of omega-(alpha-bromoketo) octanals and nonanal with oxygenated functions and formation of the corresponding eight-membered carbocyclic aldols by subsequent samarium(II)-mediated cyclization are demonstrated. Cyclooctenones deoxygenated at the C2 or C10 position in the taxane framework are prepared by dehydration
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service