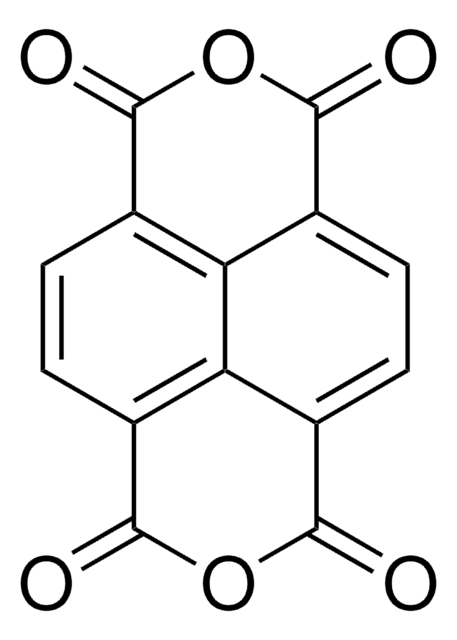
B9750
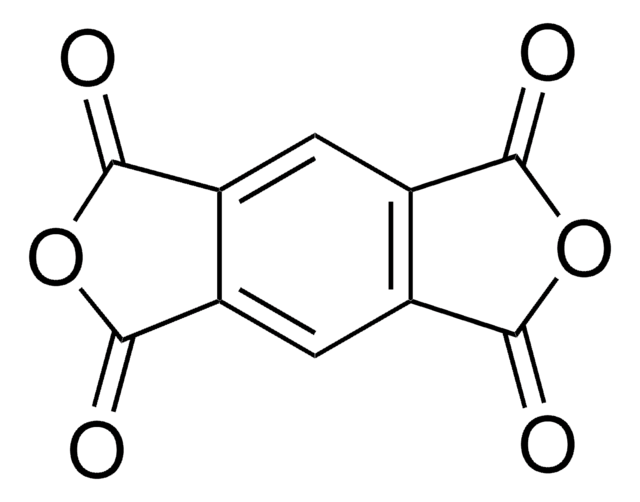
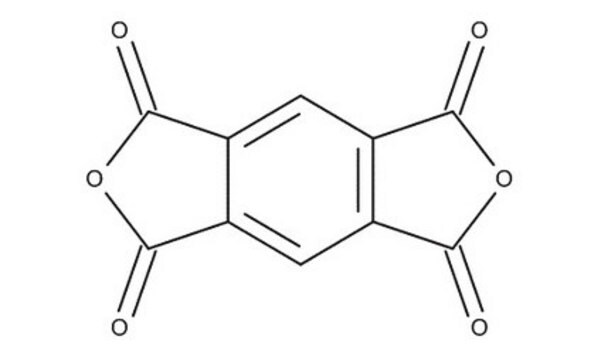
Benzophenone-3,3′,4,4′-tetracarboxylic dianhydride
98%
Synonym(s):
4,4′-Carbonyldiphthalic anhydride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C17H6O7
CAS Number:
Molecular Weight:
322.23
Beilstein:
761777
EC Number:
MDL number:
UNSPSC Code:
12162002
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
vapor density
1.4 (vs air)
Quality Level
vapor pressure
<0.1 mmHg ( 0 °C)
Assay
98%
form
powder
autoignition temp.
975 °F
expl. lim.
16 %
mp
218-222 °C (lit.)
SMILES string
O=C1OC(=O)c2cc(ccc12)C(=O)c3ccc4C(=O)OC(=O)c4c3
InChI
1S/C17H6O7/c18-13(7-1-3-9-11(5-7)16(21)23-14(9)19)8-2-4-10-12(6-8)17(22)24-15(10)20/h1-6H
InChI key
VQVIHDPBMFABCQ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Benzophenone-3,3′,4,4′-tetracarboxylic acid is formed by hydrolysis, it can be converted to the dianhydride by treating with Ac2O (molar ratio 4 to 1 of acid).The dianhydride is used to synthesize polyimides, which are highly flexible in nature due to the presence of keto and carbonyl groups in the dianhydride molecule. The keto and carbonyl groups subsequently increase the spacing between the imide rings enhancing the solubility and processibility.
Application
Used in the synthesis of polyimides.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Armarego WLF and Chai C
Purification of Laboratory Chemicals (2012)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service