B90602
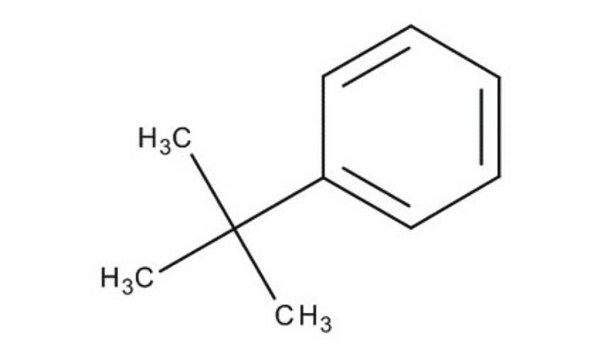
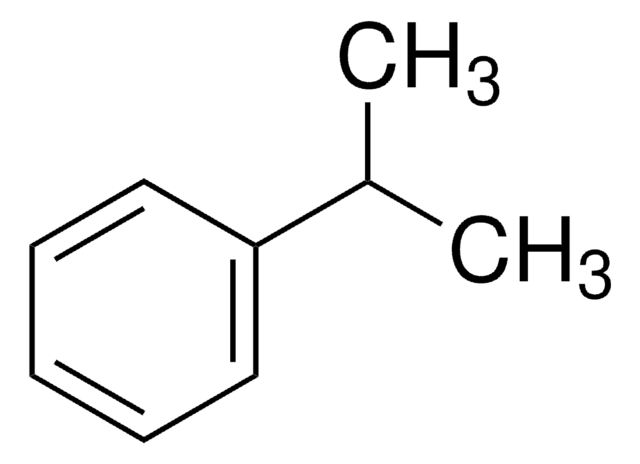
tert-Butylbenzene
99%
Synonym(s):
2-Methyl-2-phenylpropane
About This Item
Recommended Products
vapor density
3.16 (169 °C, vs air)
Quality Level
vapor pressure
4.79 mmHg ( 37.7 °C)
Assay
99%
form
liquid
autoignition temp.
842 °F
refractive index
n20/D 1.492 (lit.)
bp
169 °C (lit.)
mp
−58 °C (lit.)
density
0.867 g/mL at 25 °C (lit.)
SMILES string
CC(C)(C)c1ccccc1
InChI
1S/C10H14/c1-10(2,3)9-7-5-4-6-8-9/h4-8H,1-3H3
InChI key
YTZKOQUCBOVLHL-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- Gas separation applications: tert-Butylbenzene is utilized in the synthesis of aromatic polyimide membranes with covalent crosslinking, enhancing their performance in gas separation processes, which is vital for industrial applications and environmental management (Lozano et al., 2022).
- Enhancement of Raman scattering: The compound serves as a substrate for metal nanoparticles in nanogap-enhanced Raman scattering studies, which is crucial for analytical chemistry, providing sensitive detection methods for various chemicals (Chen et al., 2021).
- Hyperpolarized (13)C probes: It is also pivotal in the rational design of hyperpolarized (13)C probes, enabling significant advancements in magnetic resonance imaging (MRI) techniques, which improve diagnostic imaging capabilities (Sando et al., 2018).
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 3 - Skin Irrit. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point(F)
116.6 °F - closed cup
Flash Point(C)
47 °C - closed cup
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Protocols
US EPA Method 8260 describes the analysis of volatile organic compounds in solid wastes and ground waters. This application illustrates the analysis of many compounds commonly analyzed by this method using purge and trap coupled to GC-MS.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service