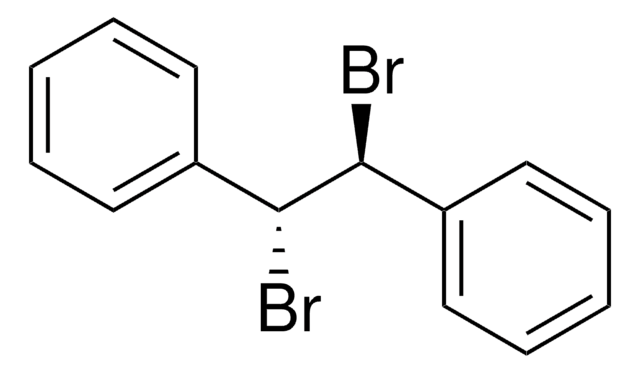
B33706
Bibenzyl
ReagentPlus®, 99%
Synonym(s):
1,2-Diphenylethane, Dibenzyl
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C6H5CH2CH2C6H5
CAS Number:
Molecular Weight:
182.26
Beilstein:
508068
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
product line
ReagentPlus®
Assay
99%
form
crystals
autoignition temp.
896 °F
bp
284 °C (lit.)
mp
50-53 °C (lit.)
density
1.014 g/mL at 25 °C (lit.)
SMILES string
C(Cc1ccccc1)c2ccccc2
InChI
1S/C14H14/c1-3-7-13(8-4-1)11-12-14-9-5-2-6-10-14/h1-10H,11-12H2
InChI key
QWUWMCYKGHVNAV-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Bibenzyl is used in the preparation of flame-retardant, high-density rigid polyurethane foams. Additionally, it can also be used to synthesize acetylcholinesterase (AChE) and butyrylcholinesterase (BuChE) inhibitors.
Legal Information
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
230.0 °F - closed cup
Flash Point(C)
110 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Flame?retardant and mechanical properties of high?density rigid polyurethane foams filled with decabrominated dipheny ethane and expandable graphite.
Ye L, et al.
Journal of Applied Polymer Science, 111(5), 2372-2380 (2009)
Design, synthesis, and biological evaluation of a new series of biphenyl/bibenzyl derivatives functioning as dual inhibitors of acetylcholinesterase and butyrylcholinesterase.
Wang D M, et al.
Molecules (Basel), 22(1), 172-172 (2017)
K H Fritzemeier et al.
European journal of biochemistry, 133(3), 545-550 (1983-07-01)
Hydroxy derivatives of 9,10-dihydrophenanthrenes, orchinol and hircinol, were isolated from bulbs of Orchidaceae which had been induced to accumulate phytoalexins. Incorporation of radioactive precursors, L-phenylalanine and various hydroxycinnamic acids, has been investigated by feeding experiments in vivo. m-Coumaric acid and
Evan G Buchanan et al.
The Journal of chemical physics, 138(6), 064308-064308 (2013-02-22)
The spectroscopy of two flexible hydrocarbons, 1,2-diphenylethane (DPE) and 2,2,2-paracyclophane (TCP) is presented, and a predictive theoretical model for describing the alkyl CH stretch region of these hydrocarbons is developed. Ultraviolet hole-burning spectroscopy identified two isomers of DPE and a
Geunhyeong Jo et al.
Magnetic resonance in chemistry : MRC, 49(6), 374-377 (2011-04-01)
Resveratrol is a polyphenol isolated from many natural sources including grapes, mulberries, eucalyptus, spruce, lilies, and peanuts. The hydroxyl groups in polyphenols can be substituted with various functional groups, allowing production of multiple derivatives. NMR spectroscopy is used to identify
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service