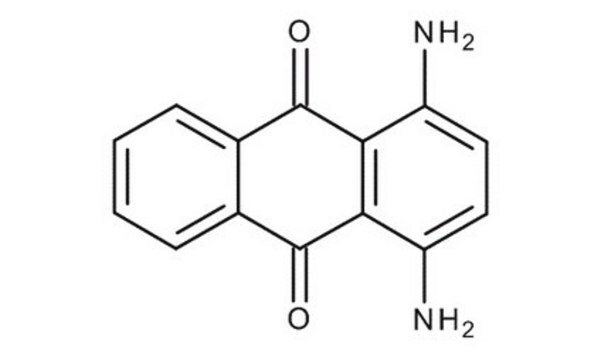
A89502
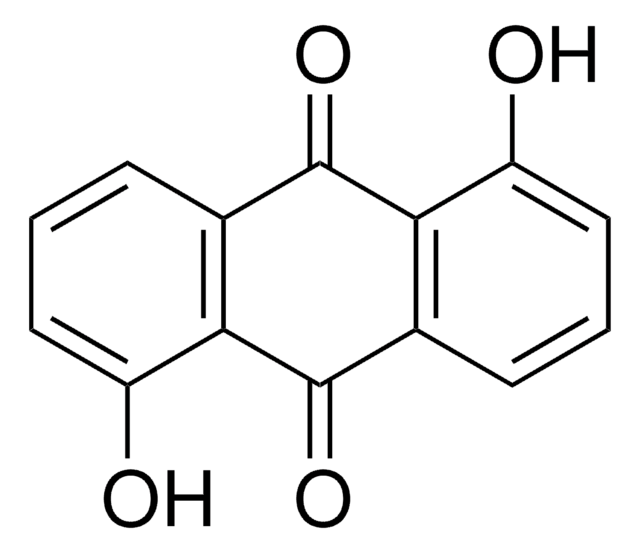
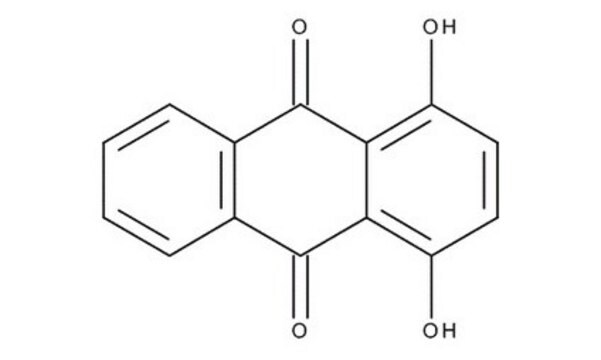
Anthraflavic acid
technical grade, 90%
Synonym(s):
2,6-Dihydroxyanthraquinone, Anthraflavine
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C14H8O4
CAS Number:
Molecular Weight:
240.21
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
technical grade
Quality Level
Assay
90%
form
powder
mp
>320 °C (lit.)
SMILES string
Oc1ccc2C(=O)c3cc(O)ccc3C(=O)c2c1
InChI
1S/C14H8O4/c15-7-1-3-9-11(5-7)14(18)10-4-2-8(16)6-12(10)13(9)17/h1-6,15-16H
InChI key
APAJFZPFBHMFQR-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Anthraflavic acid can be used as a starting material to synthesize tetrahydroxy tetrathiafulvalene (TTF) derivatives, which are used as redox-active building blocks in supramolecular and materials science. It is also utilized to prepare phosphanylidene anthra[2,1-b]furans by reacting with dialkyl acetylenedicarboxylates and triphenylphosphine.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Jie Liu et al.
Nature communications, 6, 10032-10032 (2015-12-02)
The integration of high charge carrier mobility and high luminescence in an organic semiconductor is challenging. However, there is need of such materials for organic light-emitting transistors and organic electrically pumped lasers. Here we show a novel organic semiconductor, 2,6-diphenylanthracene
A D Ayrton et al.
Mutation research, 207(3-4), 121-125 (1988-03-01)
The ability of anthraflavic acid to inhibit the mutagenicity of IQ was investigated using the Ames test and employing hepatic activation systems from Aroclor 1254-pretreated rats. Incorporation of anthraflavic acid into the S9 mix caused a concentration-dependent decrease in the
A Rodriguez et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 75(1), 479-485 (2009-12-08)
Semiconducting molecular-material thin-films of tetrabenzo (b,f,j,n) [1,5,9,13] tetraazacyclohexadecine copper(II) and nickel(II) bisanthraflavates have been prepared by using vacuum thermal evaporation on Corning glass substrates and crystalline silicon wafers. The films thus obtained were characterized by infrared spectroscopy (FTIR), atomic force
Extrapolation of in vitro antimutagenicity to the in vivo situation: the case for anthraflavic acid.
C Ioannides et al.
Basic life sciences, 61, 103-110 (1993-01-01)
H Mukhtar et al.
Cancer research, 48(9), 2361-2365 (1988-05-01)
Our recent studies have shown that naturally occurring dietary plant phenols such as tannic acid, quercetin, myricetin, and anthraflavic acid are capable of inhibiting polycyclic aromatic hydrocarbon (PAH) metabolism and subsequent PAH-DNA adduct formation in epidermis of SENCAR mice (M.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service