93690
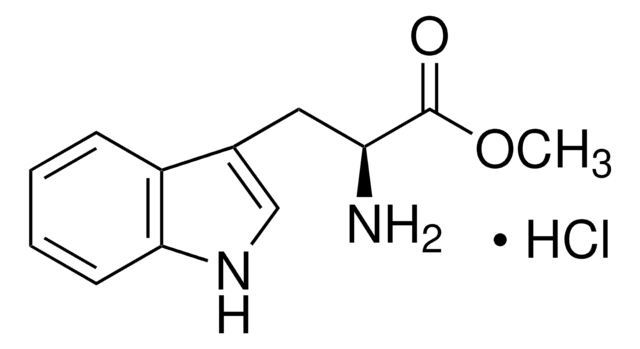
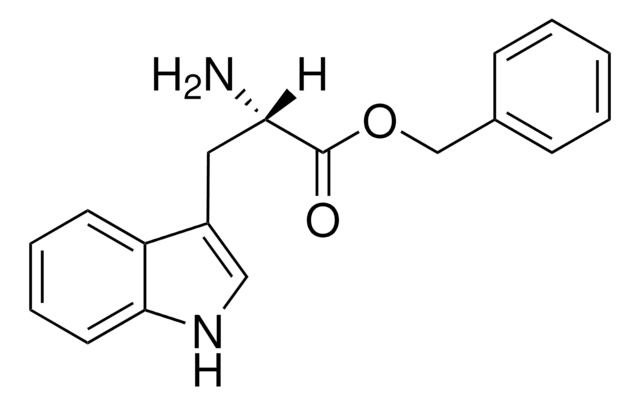
L-Tryptophan ethyl ester hydrochloride
≥99.0% (AT)
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C13H16N2O2 · HCl
CAS Number:
Molecular Weight:
268.74
Beilstein:
3919010
EC Number:
MDL number:
UNSPSC Code:
12352209
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥99.0% (AT)
form
powder
optical activity
[α]20/D +10±1°, c = 2% in H2O
reaction suitability
reaction type: solution phase peptide synthesis
mp
220-225 °C (dec.)
application(s)
peptide synthesis
SMILES string
Cl.CCOC(=O)[C@@H](N)Cc1c[nH]c2ccccc12
InChI
1S/C13H16N2O2.ClH/c1-2-17-13(16)11(14)7-9-8-15-12-6-4-3-5-10(9)12;/h3-6,8,11,15H,2,7,14H2,1H3;1H/t11-;/m0./s1
InChI key
PESYCVVSLYSXAK-MERQFXBCSA-N
Looking for similar products? Visit Product Comparison Guide
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Kento Takayama et al.
Journal of natural medicines, 75(1), 116-128 (2020-10-21)
Indole is produced from dietary tryptophan by tryptophanase in intestinal bacteria, such as Escherichia coli. In the liver, indole is converted into indoxyl sulfate, a uremic toxin and risk factor for chronic kidney disease (CKD). Probiotics and prebiotics are currently
R P Kwok et al.
Neuroendocrinology, 45(4), 267-273 (1987-04-01)
In this study we investigated the effect of insulin on striatal 5-hydroxytryptamine and tryptamine in streptozotocin-diabetic and in normal rats. Streptozotocin-diabetic rats show a reduction in rat striatal tryptophan, 5-hydroxytryptamine, and 5-hydroxyindole acetic acid, an effect observed at 7 or
A substance P antagonist improves outcome in female Sprague Dawley rats following diffuse traumatic brain injury.
Frances Corrigan et al.
CNS neuroscience & therapeutics, 18(6), 513-515 (2012-06-08)
V Iu Shviadas et al.
Biokhimiia (Moscow, Russia), 45(5), 829-834 (1980-05-01)
The dependence of the rate of spontaneous hydrolysis of tryptophan ethyl ester within a wide range of pH (4,6-10,3) was studied. This dependence was found to differ from other dependences, i.e. within the pH range of 4,6-7,0 the value of
Use of ethyl esters of tryptophan to bypass the absorption defect in Hartnup disease.
Nutrition reviews, 48(1), 22-24 (1990-01-01)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service