902578
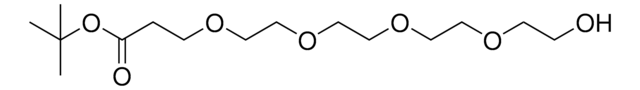
Hydroxy-PEG6-t-butyl ester
≥95%
Synonym(s):
tert-Butyl 1-hydroxy-3,6,9,12,15,18-hexaoxahenicosan-21-oate, HO-PEG6-CO-OtBu
About This Item
Recommended Products
Assay
≥95%
form
liquid
reaction suitability
reagent type: cross-linking reagent
refractive index
n/D 1.454
density
1.068
functional group
ester
hydroxyl
storage temp.
−20°C
SMILES string
O=C(OC(C)(C)C)CCOCCOCCOCCOCCOCCOCCO
InChI
1S/C19H38O9/c1-19(2,3)28-18(21)4-6-22-8-10-24-12-14-26-16-17-27-15-13-25-11-9-23-7-5-20/h20H,4-17H2,1-3H3
InChI key
VGGDPFAYSOSIOK-UHFFFAOYSA-N
Application
Other Notes
Maleimide-oligo(ethylene glycol) Derivatives of Camptothecin as Albumin-Binding Prodrugs: Synthesis and Antitumor Efficacy
Legal Information
related product
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service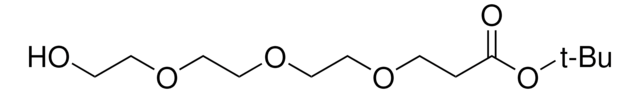

![2-[2-(2-Aminoethoxy)ethoxy]ethanol ≥96.0% (GC)](/deepweb/assets/sigmaaldrich/product/structures/237/185/b94eadd2-5a2c-4a5b-a13d-3d3905df0dbc/640/b94eadd2-5a2c-4a5b-a13d-3d3905df0dbc.png)