900563
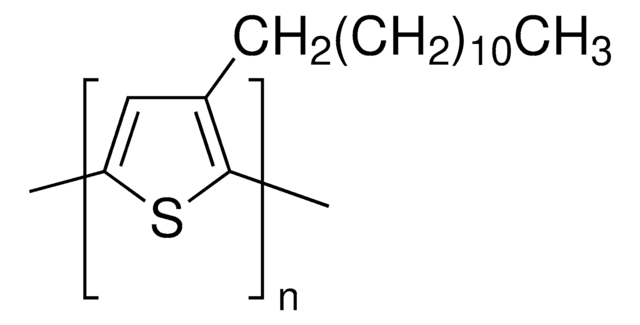
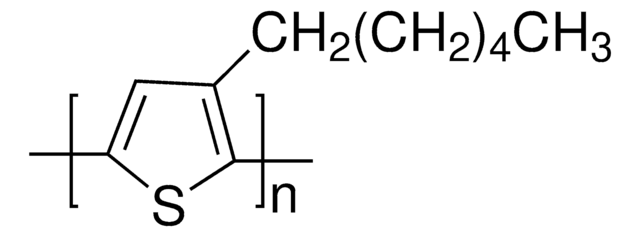
Poly(3-hexylthiophene-2,5-diyl)
regioregular, average Mw 20,000-45,000
Synonym(s):
P3HT
About This Item
Recommended Products
description
Metal purity: Mg: ≤100 ppm
Ni: ≤100 ppm
Regioregularity: ≥90%
Quality Level
form
solid
mol wt
average Mw 20,000-45,000
InChI
1S/C12H20S/c1-4-5-6-7-8-12-9-10(2)13-11(12)3/h9H,4-8H2,1-3H3
InChI key
DUFPJSOXRHVDOV-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- Active semiconductor to prepare organic field emission transistors. In regioregular P3HT the microcrystalline domains are well constructed by phase-segregated layers of insulating alkali side chains and pi-pi interchain stacking. This leads to high charge mobility and enhances the performance of OFETs.
- Precursor to synthesize P3HT-PCBM( [6,6]-phenyl C61-butyric acid methyl ester) blend thin film. The highcrystallinity of P3HT thin film increases the efficiency of organicphotovoltaic cells.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Professor Shinar (Iowa State University, USA) summarizes the developments of a variety of sensor configurations based on organic and hybrid electronics, as low-cost, disposable, non-invasive, wearable bioelectronics for healthcare.
Intrinsically stretchable active layers for organic field-effect transistors (OFET) are discussed. Polymer structural modification & post-polymerization modifications are 2 methods to achieve this.
Next generation solar cells have the potential to achieve conversion efficiencies beyond the Shockley-Queisser (S-Q) limit while also significantly lowering production costs.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![[6,6]-Phenyl C61 butyric acid methyl ester ≥99%](/deepweb/assets/sigmaaldrich/product/structures/359/221/d990c746-0960-4c69-bf76-fe09b193824d/640/d990c746-0960-4c69-bf76-fe09b193824d.png)
![[6,6]-Phenyl C71 butyric acid methyl ester, mixture of isomers 99%](/deepweb/assets/sigmaaldrich/product/structures/716/624/9fb9f2f0-ae99-429f-8d3a-b12267976a4d/640/9fb9f2f0-ae99-429f-8d3a-b12267976a4d.png)