857270
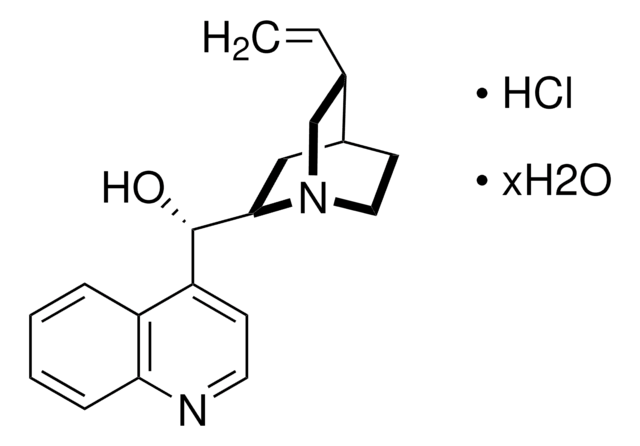
(+)-Cinchonine
85%
Synonym(s):
Cinchonine monohydrochloride dihydrate, NSC 6176
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C19H22N2O
CAS Number:
Molecular Weight:
294.39
Beilstein:
89689
EC Number:
MDL number:
UNSPSC Code:
12352104
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
85%
form
solid
optical activity
[α]23/D +228°, c = 0.5 in ethanol
mp
258-260 °C (lit.)
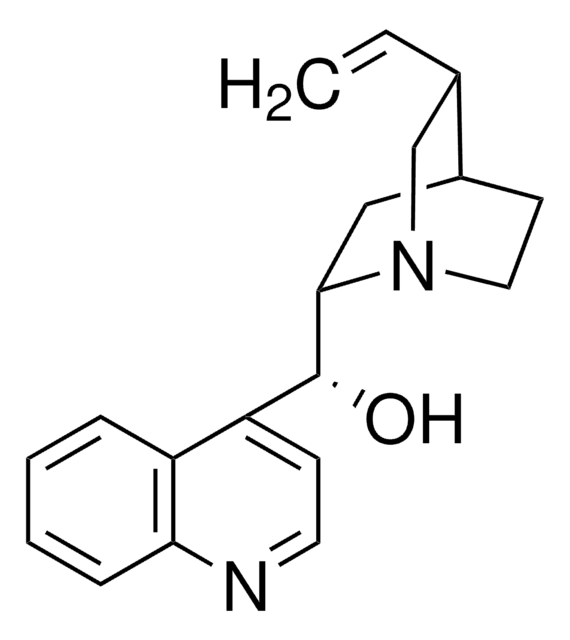
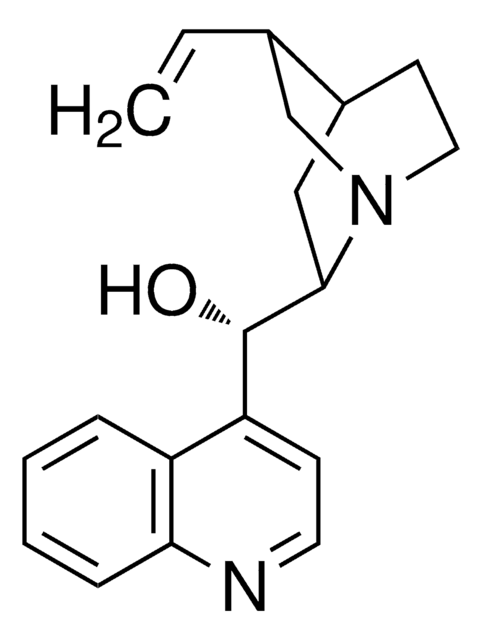
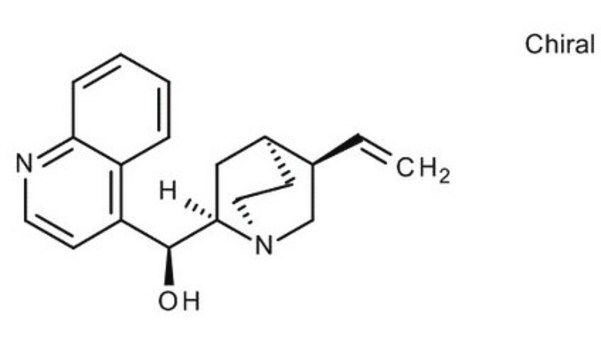
SMILES string
[H][C@@]12CCN(C[C@H]1C=C)[C@]([H])(C2)[C@@H](O)c3ccnc4ccccc34
InChI
1S/C19H22N2O/c1-2-13-12-21-10-8-14(13)11-18(21)19(22)16-7-9-20-17-6-4-3-5-15(16)17/h2-7,9,13-14,18-19,22H,1,8,10-12H2/t13-,14-,18+,19-/m0/s1
InChI key
KMPWYEUPVWOPIM-QAMTZSDWSA-N
General description
(+)-Cinchonine, one of the alkaloids found in the barks of cinchona tree, is mainly used in the treatment of malaria. It belongs to the monoclinic crystal system and P21 space group. The solubility of cinchonine can be improved by the formation of inclusion complexes with cyclodextrins.
Application
(+)-Cinchonine, in the presence of lithium diisopropylamide (LDA) forms a complex, which can catalyze the asymmetric conjugate addition of benzyl- and alkylphosphonates to aromatic and heteroaromatic nitroalkenes to form the corresponding adducts.
Other Notes
remainder dihydrocinchonine
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Skin Sens. 1A
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Cinchonine catalyzed diastereo-and enantioselective Michael addition of a-lithiated phosphonates to nitroalkenes.
Rai V, et al.
Tetrahedron Asymmetry, 18(22), 2719-2726 (2007)
Song CE.
Cinchona Alkaloids in Synthesis and Catalysis: Ligands, Immobilization and Organocatalysis, 2-3 (2009)
The molecular and crystal structure of the alkaloid cinchonine.
Oleksyn B, et al.
Acta Crystallographica Section B, Structural Science, 35(2), 440-444 (1979)
Qing Gu et al.
Organic letters, 13(19), 5192-5195 (2011-09-15)
Desymmetrization of cyclohexadienones bearing a bisphenylsulfonyl methylene group via asymmetric Michael reaction catalyzed by cinchonine-derived urea was realized to afford a series of highly enantioenriched polycyclic cyclohexenones in high yields and ee's.
Yu-Hua Liao et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 18(21), 6679-6687 (2012-04-14)
An asymmetric conjugate addition of 3-monosubstituted oxindoles to a range of (E)-1,4-diaryl-2-buten-1,4-diones, catalyzed by commercially available cinchonine, is described. This organocatalytic asymmetric reaction affords a broad range of 3,3'-disubstituted oxindoles that contain a 1,4-dicarbonyl moiety and vicinal quaternary and tertiary
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service