All Photos(2)
About This Item
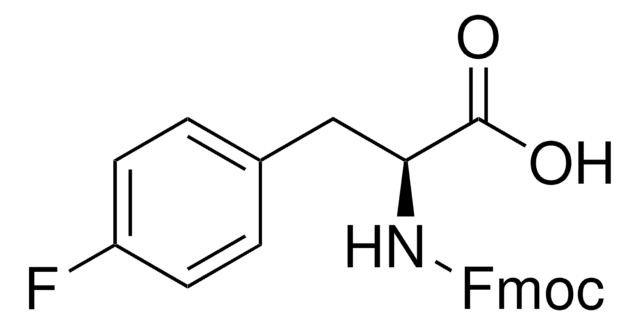
Linear Formula:
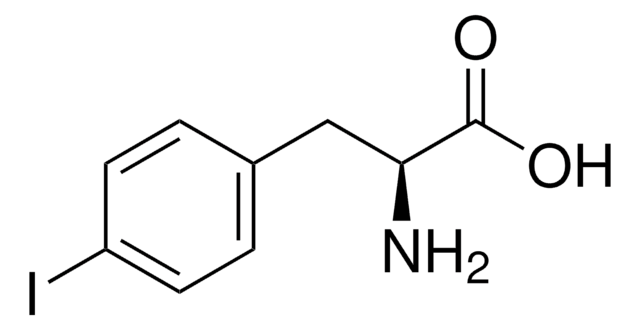
BrC6H4CH2CH(NH2)CO2H
CAS Number:
Molecular Weight:
244.09
EC Number:
MDL number:
UNSPSC Code:
12352209
eCl@ss:
32160406
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
powder
reaction suitability
reaction type: solution phase peptide synthesis
mp
262-263 °C (dec.) (lit.)
application(s)
peptide synthesis
storage temp.
2-8°C
SMILES string
NC(Cc1ccc(Br)cc1)C(O)=O
InChI
1S/C9H10BrNO2/c10-7-3-1-6(2-4-7)5-8(11)9(12)13/h1-4,8H,5,11H2,(H,12,13)
InChI key
PEMUHKUIQHFMTH-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Inchan Kwon et al.
Journal of the American Chemical Society, 128(36), 11778-11783 (2006-09-07)
Introduction of a yeast suppressor tRNA (ytRNA(Phe)(CUA)) and a mutant yeast phenylalanyl-tRNA synthetase (yPheRS (T415G)) into an Escherichia coli expression host allows in vivo incorporation of phenylalanine analogues into recombinant proteins in response to amber stop codons. However, high-fidelity incorporation
Neutron capture therapy for melanoma.
J A Coderre et al.
Basic life sciences, 50, 219-232 (1989-01-01)
Thermal neutron capture therapy: the Japanese-Australian clinical trial for malignant melanoma.
B J Allen et al.
Basic life sciences, 50, 69-73 (1989-01-01)
James M Turner et al.
Proceedings of the National Academy of Sciences of the United States of America, 103(17), 6483-6488 (2006-04-19)
Recently, tRNA aminoacyl-tRNA synthetase pairs have been evolved that allow one to genetically encode a large array of unnatural amino acids in both prokaryotic and eukaryotic organisms. We have determined the crystal structures of two substrate-bound Methanococcus jannaschii tyrosyl aminoacyl-tRNA
G Basu et al.
Biochemistry, 32(12), 3067-3076 (1993-03-30)
The very strong helical propensity of peptides rich in alpha-aminoisobutyric acid (Aib) has enabled the design of a set of helices containing as guest amino acids one fluorescent chromophore, beta-(1'-naphthyl)-L-alanine, and one heavy atom perturber, p-bromo-L-phenylalanine. The fluorescence of the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service