850993
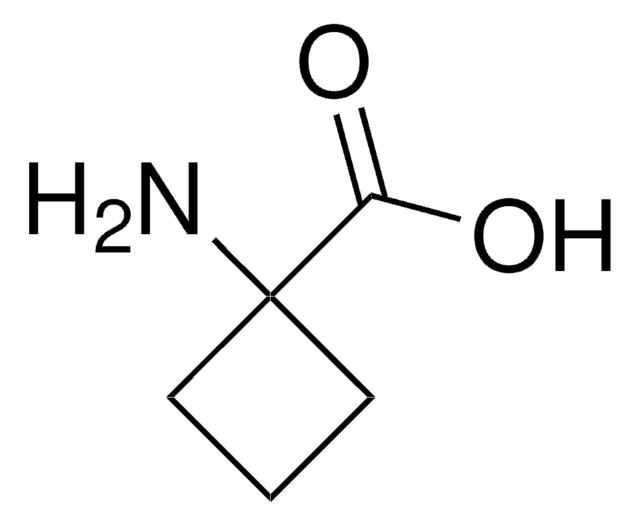
2-Aminoisobutyric acid
98%, for peptide synthesis
Synonym(s):
α-Aminoisobutyric acid, 2-Methylalanine, Aib
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(CH3)2C(NH2)COOH
CAS Number:
Molecular Weight:
103.12
Beilstein:
506496
EC Number:
MDL number:
UNSPSC Code:
12352106
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
product name
2-Aminoisobutyric acid, 98%
Quality Level
Assay
98%
form
solid
reaction suitability
reaction type: solution phase peptide synthesis
mp
≥300 °C
application(s)
peptide synthesis
SMILES string
CC(C)(N)C(O)=O
InChI
1S/C4H9NO2/c1-4(2,5)3(6)7/h5H2,1-2H3,(H,6,7)
InChI key
FUOOLUPWFVMBKG-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
2-Aminoisobutyric acid also known as α-aminobutyric acid, is an amino acid used in solution-phase peptide synthesis. It is a desirable building block for peptides because of its strong tendency to cause the peptide to form a helical shape.
Application
2-Aminoisobutyric acid can be used to synthesize self-assembled polypeptide nanoparticles. Incorporation of this compound into the peptide chain can prevent undesired reactions since it is di-α-substituted, and inert to C−H abstraction.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
C Lucarelli et al.
Journal of chromatography, 541(1-2), 285-296 (1991-03-22)
A procedure is described for the determination of alpha-methyldopa (MD) [L-3-(3,4-dihydroxyphenyl)-2-methylalanine], its metabolite and catecholamines in the urine and plasma of patients undergoing MD therapy, by high-performance liquid chromatography with dual working electrode coulometric detection. An efficient sample preparation procedure
Yutong Yang et al.
Membranes, 10(11) (2020-11-14)
Amine-containing mixed-matrix membranes incorporated with amino-functionalized multi-walled carbon nanotubes (AF-MWNTs) were synthesized for CO2/H2 separation based on the facilitated transport mechanism. AF-MWNTs were chosen primarily as the mechanical reinforcing filler to enhance the membrane stability. At 107 °C and 0.2-MPa
Lucia Becucci et al.
Journal of the American Chemical Society, 132(17), 6194-6204 (2010-04-16)
Four oligopeptides consisting of a sequence of alpha-aminoisobutyric acid (Aib) residues, thiolated at either the N- or C-terminus by means of a -(CH(2))(2)-SH anchor, were self-assembled on mercury, which is a substrate known to impart a high fluidity to self-assembled
Øyvind Jacobsen et al.
The Journal of organic chemistry, 76(5), 1228-1238 (2011-02-01)
Short peptides are important as lead compounds and molecular probes in drug discovery and chemical biology, but their well-known drawbacks, such as high conformational flexibility, protease lability, poor bioavailability and short half-lives in vivo, have prevented their potential from being
Anne Goj et al.
The Journal of chemical physics, 134(20), 205103-205103 (2011-06-07)
We use mixed classical/quantum simulations to study the time dependence of an excitation of a C=O vibration on a 3-10 helix of α-aminoisobutyric acid, a system which represents a test case for the formation of self-trapped vibrational excitation states on
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service