798444
Endo-4-Methoxyphenyl Kwon [2.2.1] Bicyclic Phosphine
Synonym(s):
(1S,4S,5S)-5-(4-methoxyphenyl)-2-tosyl-2-aza-5-phosphabicyclo[2.2.1]heptane
About This Item
Recommended Products
form
powder
Quality Level
mp
156-161 °C
storage temp.
2-8°C
SMILES string
O=S(N1C[C@H]2[P@@](C3=CC=C(OC)C=C3)C[C@@H]1C2)(C4=CC=C(C)C=C4)=O
InChI
1S/C19H22NO3PS/c1-14-3-9-19(10-4-14)25(21,22)20-12-18-11-15(20)13-24(18)17-7-5-16(23-2)6-8-17/h3-10,15,18H,11-13H2,1-2H3
InChI key
RMFZMNUGIFSNIE-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Other Notes
Technology Spotlight- Kwon Phosphines: P-Chiral Monodentate Phosphines from Hydroxyproline
Aldrichimica Acta Review- Nucleophilic Chiral Phosphines: Powerful and Versatile Catalysts for Asymmetric Annulations
related product
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Chiral phosphines have been the staple ligands for asymmetric transition metal catalysis and more recently operate as catalysts in organic phosphinocatalysis.
Related Content
The Kwon Group has made major strides in the development and application of nucleophilic phosphinocatalysis reactions as a means to synthesize carbo- and heterocycles that serve as synthetic intermediates for both natural products and medicinally useful compounds.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service![Exo-Phenyl Kwon [2.2.1] Bicyclic Phosphine 95% (HPLC)](/deepweb/assets/sigmaaldrich/product/structures/477/026/5255f657-4af5-47da-9839-86b94d92129f/640/5255f657-4af5-47da-9839-86b94d92129f.png)
![Exo-4-anisole Kwon [2.2.1] bicyclic phosphine](/deepweb/assets/sigmaaldrich/product/structures/114/753/2a544671-b0e0-4556-8dc3-46c126d6c8ab/640/2a544671-b0e0-4556-8dc3-46c126d6c8ab.png)
![Endo-Phenyl Kwon [2.2.1] Bicyclic Phosphine 95% (HPLC)](/deepweb/assets/sigmaaldrich/product/structures/207/060/39f2b621-f484-49c3-b692-cdb610e8c517/640/39f2b621-f484-49c3-b692-cdb610e8c517.png)
![Exo-2-Naphthyl Kwon [2.2.1] Bicyclic Phosphine](/deepweb/assets/sigmaaldrich/product/structures/324/907/a6c29ce5-9be1-4585-9bfb-6dca8272f6e4/640/a6c29ce5-9be1-4585-9bfb-6dca8272f6e4.png)
![Endo-1-Naphthyl Kwon [2.2.1] Bicyclic Phosphine](/deepweb/assets/sigmaaldrich/product/structures/170/551/a39b471a-5427-43d5-9420-111b638ec1ac/640/a39b471a-5427-43d5-9420-111b638ec1ac.png)
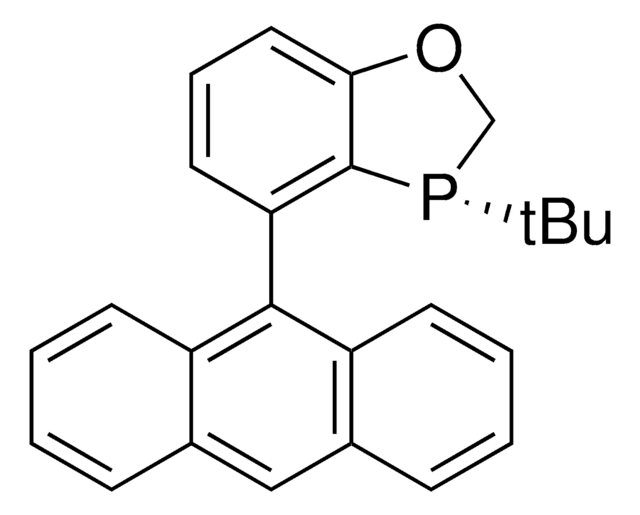
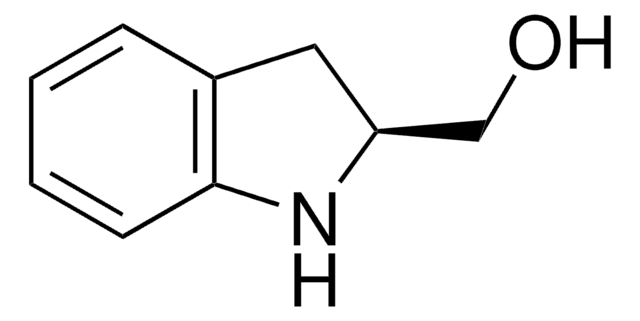
![(R)-(–)-4,12-Bis(diphenylphosphino)-[2.2]-paracyclophane 96%](/deepweb/assets/sigmaaldrich/product/structures/131/143/7e18cd49-a90e-4d89-a189-4f37ad9e6cd2/640/7e18cd49-a90e-4d89-a189-4f37ad9e6cd2.png)
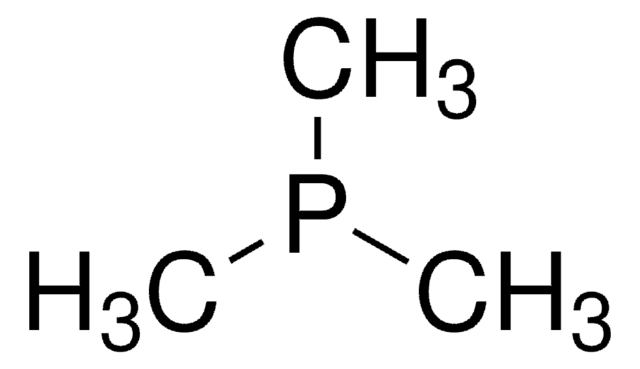
![Exo-1-Naphthyl Kwon [2.2.1] Bicyclic Phosphine](/deepweb/assets/sigmaaldrich/product/structures/376/476/5be457d0-df63-4a3d-823c-9a8002a7a813/640/5be457d0-df63-4a3d-823c-9a8002a7a813.png)
