757322
3,6-Di-tert-butylcarbazole
97%
Synonym(s):
3,6-Di-tert-butyl-9H-carbazole
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C20H25N
CAS Number:
Molecular Weight:
279.42
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Assay
97%
form
powder
mp
228-233
SMILES string
CC(C)(C)c1ccc2[nH]c3ccc(cc3c2c1)C(C)(C)C
InChI
1S/C20H25N/c1-19(2,3)13-7-9-17-15(11-13)16-12-14(20(4,5)6)8-10-18(16)21-17/h7-12,21H,1-6H3
InChI key
OYFFSPILVQLRQA-UHFFFAOYSA-N
Related Categories
General description
3,6-Di-tert-butylcarbazole is a carbazole based material with hole transporting characteristics. The 3,6-Di-tert-butyl component of the carbazole results in an increase in the glass transition temperature (Tg) of the compound. It can be used in combination with another carbazole to form novel electroluminescent materials.
Application
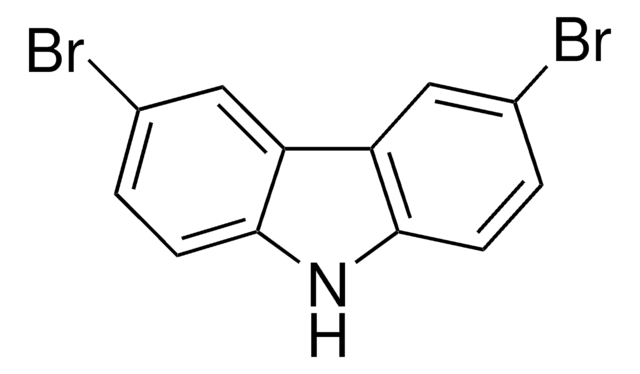
3,6-Di-tert-butylcarbazole is mainly used as a monomeric precursor in the syntheses of new carbazole based materials which consist of ethynylphenyl. These materials include 9-(4-bromophenyl)-3,6-di-tert-butylcarbazol and 2-(4-(2-(4-(3,6-di-tert-butyl-9H-carbazol-9-yl)phenyl)ethynyl)benzylidene)malononitrile (PBM) which can be further be used in organic light emitting diodes (OLEDs) and optical switching devices.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Carbazole based hole transporting materials for solid state dye sensitizer solar cells: role of the methoxy groups.
Degbia M, et al.
Polymer International, 63(8), 1387-1393 (2014)
Solvent dependant optical switching in carbazole-based fluorescent nanoparticles.
Adhikari RM, et al.
Langmuir, 25(4), 2402-2406 (2009)
Synthesis and photophysical properties of carbazole-based blue light-emitting dendrimers.
Adhikari RM, et al.
The Journal of Organic Chemistry, 72(13), 4727-4732 (2007)
Bis (carbazolyl) derivatives of pyrene and tetrahydropyrene: synthesis, structures, optical properties, electrochemistry, and electroluminescence.
Kaafarani BR, et al.
Journal of Material Chemistry C, 1(8), 1638-1650 (2013)
Ji Won Yang et al.
Physical chemistry chemical physics : PCCP, 18(45), 31330-31336 (2016-11-09)
Bis(phenylsulfone) was developed as a strong electron acceptor of thermally activated delayed fluorescent emitters. The connection of two electron withdrawing phenylsulfone moieties through meta-position of phenyl produced the bis(phenylsulfone) acceptor and the strong electron acceptor strength of bis(phenylsulfone) enabled preparation
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service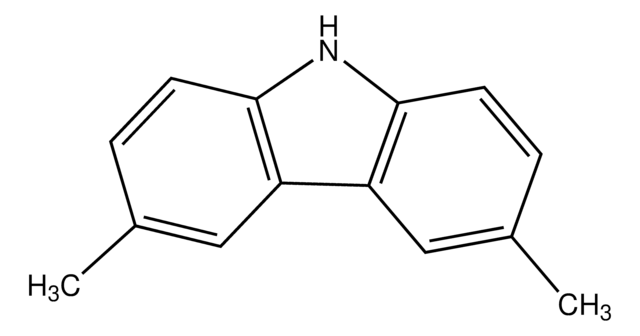


![[9-(4-Bromophenyl)]-3,6-di-tert-butyl-9H-carbazole](/deepweb/assets/sigmaaldrich/product/structures/214/779/819c00e2-ee0a-4166-977c-a7f68002b43d/640/819c00e2-ee0a-4166-977c-a7f68002b43d.png)