749141
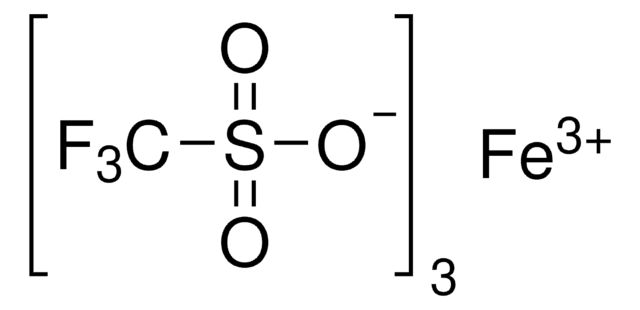
Iron(III) oxo acetate perchlorate hydrate
Synonym(s):
Hexakis[μ-(acetato-κO:κO′ )]triaqua-μ3-oxotriiron(1+) perchlorate hydrate, Trimeric Fe cluster
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C12H24Fe3O16 · ClO4 · xH2O
Molecular Weight:
691.29 (anhydrous basis)
UNSPSC Code:
26111700
NACRES:
NA.23
Recommended Products
form
powder
Quality Level
greener alternative product characteristics
Design for Energy Efficiency
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
greener alternative category
, Enabling
Related Categories
General description
We are committed to bringing you Greener Alternative Products, which adhere to one or more of The 12 Principles of Greener Chemistry. This product has been enhanced for energy efficiency. Find details here.
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Steffen Hausdorf et al.
Journal of the American Chemical Society, 132(32), 10978-10981 (2010-08-12)
A simple two-component procedure was developed to synthesize not only classical zinc-based IRMOFs represented by MOF-5 but also the cobalt and beryllium homologues of this most prominent MOF. The procedure is the first manifestation of mirroring the IRMOF series with
Steffen Hausdorf et al.
Dalton transactions (Cambridge, England : 2003), 7(7), 1107-1113 (2009-03-27)
An ethynylene diisophthalic acid linker molecule was synthesized and used to form a zinc carboxylate-based metal organic framework (MOF) with very large pores and unit cell volume resulting from the unusual combination of structurally different inorganic units forming the secondary
Steffen Hausdorf et al.
The journal of physical chemistry. A, 112(33), 7567-7576 (2008-07-26)
The contributions of terephthalic acid and Zn(2+)-coordinated water in N,N-diethylformamide (DEF) to the overall proton activity in the synthesis of MOF-5 (Zn4O(BDC)3, BDC = 1,4-benzenedicarboxylate) were quantitatively determined by combined electrochemical and UV-vis spectroscopic measurements. Structural transformations of zinc carboxylate-based
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service