740683
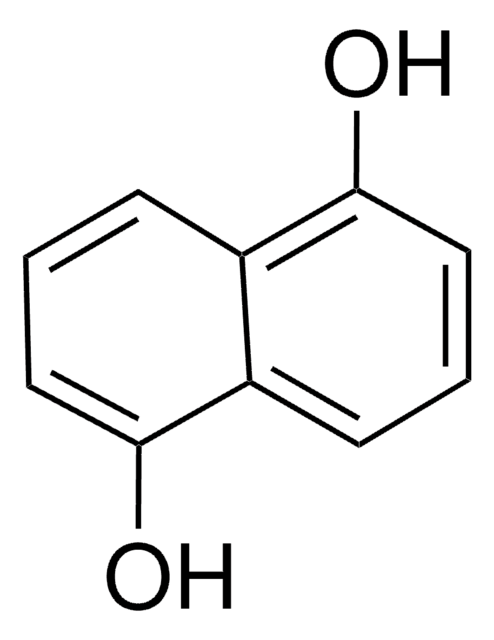
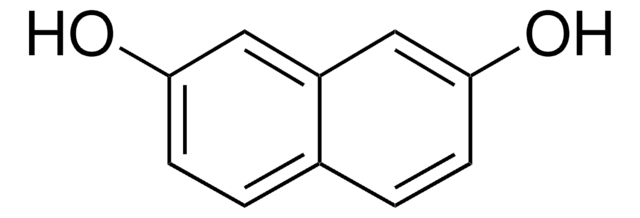
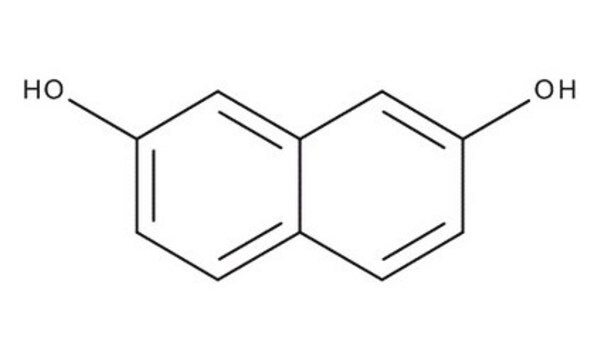
1,8-Dihydroxynaphthalene
95%
Synonym(s):
1,8-Naphthalenediol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C10H8O2
CAS Number:
Molecular Weight:
160.17
Beilstein:
2044947
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
form
solid
mp
137-143 °C
storage temp.
2-8°C
SMILES string
Oc1cccc2cccc(O)c12
InChI
1S/C10H8O2/c11-8-5-1-3-7-4-2-6-9(12)10(7)8/h1-6,11-12H
InChI key
OENHRRVNRZBNNS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
1,8-Dihydroxynaphthalene (DHN) can be used as:
- An intermediate in the preparation of benzo analogs of spiromamakone A.
- A starting material to synthesize naphthopyran derivatives.
- An intermediate in the total synthesis of palmarumycin CP17 analogs.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
E Thines et al.
The Journal of antibiotics, 51(4), 387-393 (1998-06-19)
From submerged cultures of Scytalidium sp. 36-93, ten metabolites were isolated due to their effects on dihydroxynaphthalene (DHN) or DOPA melanin biosynthesis. Four of the compounds, scytalols A (1a), B (1b), C (2) and D (3), are new secondary metabolites
Pigment biosynthesis and virulence.
A A Brakhage et al.
Contributions to microbiology, 2, 205-215 (1999-10-16)
Hong Jiang et al.
Gene, 602, 8-15 (2016-11-16)
A PKS1 gene responsible for the melanin biosynthesis and a NPG1 gene in Aureobasidium melanogenum XJ5-1 were cloned and characterized. An ORF of the PKS1 gene encoding a protein with 2165 amino acids contained 6495bp while an ORF of the
Shao Yu Lin et al.
Molecular plant-microbe interactions : MPMI, 25(12), 1552-1561 (2012-09-01)
Both Colletotrichum and Magnaporthe spp. develop appressoria pigmented with melanin, which is essential for fungal pathogenicity. 1,8-Dihydroxynaphthalene (1,8-DHN) is believed to be polymerized to yield melanin around the appresorial cell wall through the oxidative activity of laccases. However, no 1,8-DHN
H F Tsai et al.
The Journal of biological chemistry, 276(31), 29292-29298 (2001-05-15)
Chain lengths and cyclization patterns of microbial polyketides are generally determined by polyketide synthases alone. Fungal polyketide melanins are often derived from a pentaketide 1,8-dihydroxynaphthalene, and pentaketide synthases are used for synthesis of the upstream pentaketide precursor, 1,3,6,8-tetrahydroxynaphthalene (1,3,6,8-THN). However
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service