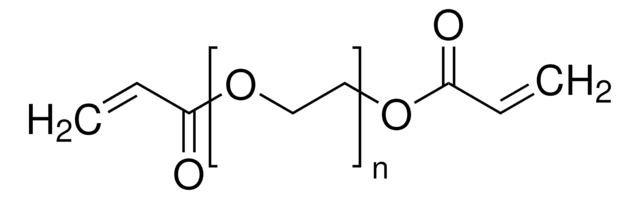
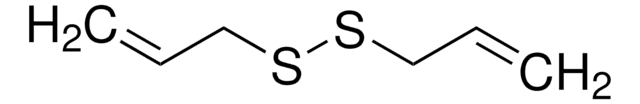735094
Bis(2-methacryloyl)oxyethyl disulfide
contains ≤6000 ppm hydroquinone as stabilizer
Synonym(s):
DSDMA, Disulfide-based dimethacrylate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C12H18O4S2
CAS Number:
Molecular Weight:
290.40
MDL number:
UNSPSC Code:
12162002
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
form
liquid
Quality Level
contains
≤6000 ppm hydroquinone as stabilizer
refractive index
n20/D 1.517
density
1.141 g/mL at 25 °C
storage temp.
2-8°C
SMILES string
CC(=C)C(=O)OCCSSCCOC(=O)C(C)=C
InChI
1S/C12H18O4S2/c1-9(2)11(13)15-5-7-17-18-8-6-16-12(14)10(3)4/h1,3,5-8H2,2,4H3
InChI key
CGDNFXSLPGLMHK-UHFFFAOYSA-N
Related Categories
General description
Bis(2-methacryloyl)oxyethyl disulfide (DSDMA) belongs to the class of monomers known as disulfide-based dimethacrylates. It is widely employed as a crosslinker in the synthesis of various polymers with specific properties such as redox sensitivity and self-healing properties. DSDMA contains a disulfide bond, which can be cleaved under specific conditions, making it useful for drug delivery systems. It also undergoes thiol-disulfide exchange reactions, allowing it to react with thiols in polymers and form covalent crosslinks. This property enables the formation of networks and gels in polymer systems. Additionally, DSDMA is employed in the development of biomedical materials, such as tissue engineering scaffolds. Its ability to form stable crosslinks in biological environments makes it suitable for these applications.
Application
Bis(2-methacryloyl)oxyethyl disulfide (DSDMA) can be used in the following applications:
- Used as a crosslinker in the synthesis of reduction-responsive molecularly imprinted polymer (MIPs) nanogels for drug delivery applications. This reduction-responsive property allows for control over drug delivery and modulation of the release properties of the MIPs.
- Used as a crosslinker in the synthesis of self-healing polymer nanocomposites via dynamic disulfide exchange reaction and crosslinking properties. These self-healing polymer nanocomposites can be used in coatings, electronics, and packaging applications.
- Used as a redox-responsive cross-linker in the synthesis of zwitterionic hydrogels for effective drug delivery. DSDMA provides structural stability, redox-responsiveness, and self-healing properties, which are essential for effective drug delivery.
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 2
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Wenwen Li; Krzysztof Matyjaszewski; Krystyna Albrecht; Martin Moller
Macromolecules, 42, 8228-8228 (2009)
Kunihiko Kobayashi et al.
ACS applied materials & interfaces, 11(1), 151-159 (2018-12-12)
Soft-robotic devices such as polymeric microgrippers offer the possibility for pick and place of fragile biological cargo in hard-to-reach conduits with potential applications in drug delivery, minimally invasive surgery, and biomedical engineering. Previously, millimeter-sized self-folding thermomagnetically responsive soft grippers have
David S Spencer et al.
Journal of polymer science. Part A, Polymer chemistry, 56(14), 1536-1544 (2019-03-25)
Crosslinked cationic nanoscale networks with hydrophobic cores are an environmentally robust alternative to self-assembled polymeric drug delivery carriers with respect to therapeutic encapsulation and stability to dilution. However, the ability to tune the degree of PEG incorporated into nanogels during
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service