713708
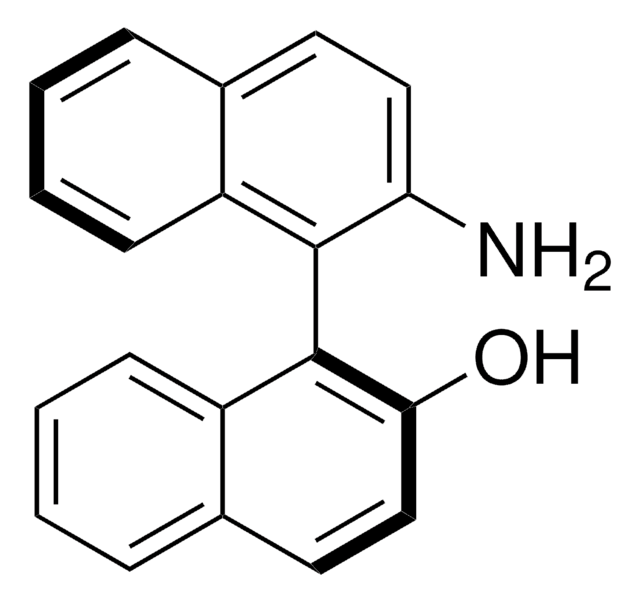
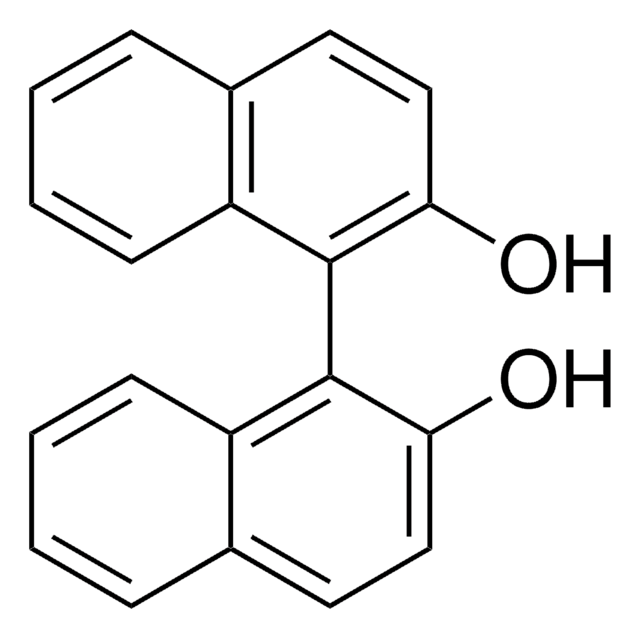
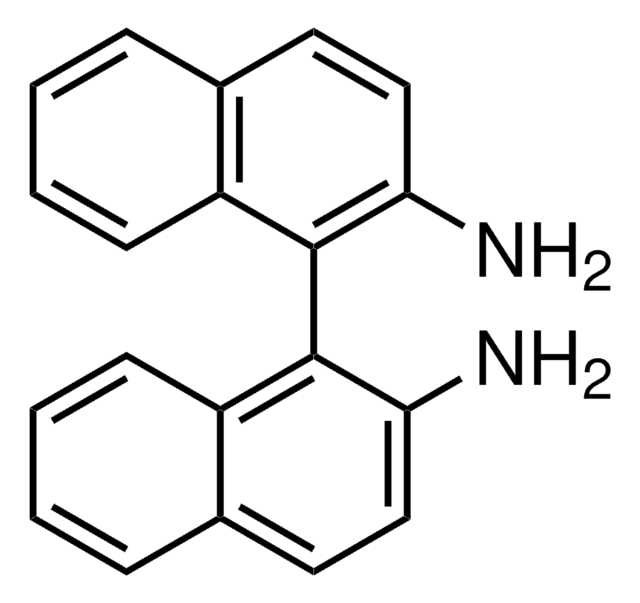
(R)-(+)-2′-Amino-1,1′-binaphthalen-2-ol
97%
Synonym(s):
(R)-(+)-2-Amino-2′-hydroxy-1,1′-binaphthalene, (R)-NOBIN
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C20H15NO
CAS Number:
Molecular Weight:
285.34
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥96.5% (HPLC)
97%
form
crystals
optical purity
enantiomeric excess: ≥98.0%
InChI
1S/C20H15NO/c21-17-11-9-13-5-1-3-7-15(13)19(17)20-16-8-4-2-6-14(16)10-12-18(20)22/h1-12,22H,21H2
InChI key
HIXQCPGXQVQHJP-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
(R)-(+)-2′-Amino-1,1′-binaphthalen-2-ol is a non-symmetrically substituted 1,1′-binaphthalene ligand.
Application
(R)-(+)-2′-Amino-1,1′-binaphthalen-2-ol may be used as a catalyst in the asymmetric PTC (phase-transfer catalysis) synthesis of α-amino acids. (R)-NOBIN reacts with trans-azobenzene-4,4′-dicarbonyl chloride to form poly(ester-amide)s, which shows backbone light-regulated chiroptical response.
Packaging
Bottomless glass bottle. Contents are inside inserted fused cone.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Acute 1 - Aquatic Chronic 1 - Eye Dam. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Stimuli-responsive polymers. 10. Photo-regulation of optical rotations in azobenzene modified poly (ester-amide) s containing highly structured, atropisomeric backbone geometries.
Lynch JG and Jaycox GD.
Polymer, 55(16), 3564-3572 (2014)
Highly Efficient Catalytic Synthesis of a-Amino Acids under Phase-Transfer Conditions with a Novel Catalyst/Substrate Pair.
Belokon YN, et al.
Angewandte Chemie (International Edition in English), 40(10), 1948-1951 (2001)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service