702471
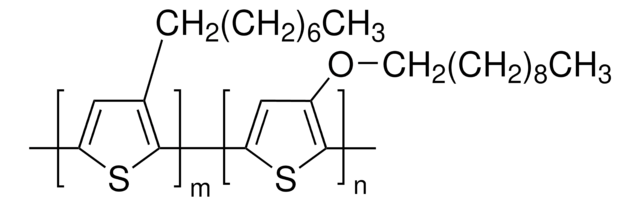
PTAA
a poly(triaryl amine) semiconductor
Synonym(s):
Poly(triaryl amine), Poly[bis(4-phenyl)(2,4,6-trimethylphenyl)amine]
About This Item
Recommended Products
Quality Level
form
solid
mol wt
average Mn 7,000-10,000 (GPC)
greener alternative product characteristics
Design for Energy Efficiency
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
mp
>400 °C
>400 °C
Mw/Mn
2‑2.2
application(s)
battery manufacturing
semiconductor
greener alternative category
, Enabling
semiconductor properties
P-type (mobility=10−3 - 10−2 cm2/V·s)
General description
Application
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Polytriarylamine Semiconductors
The development of high-performance conjugated organic molecules and polymers has received widespread attention in industrial and academic research.
Professor Shinar (Iowa State University, USA) summarizes the developments of a variety of sensor configurations based on organic and hybrid electronics, as low-cost, disposable, non-invasive, wearable bioelectronics for healthcare.
Next generation solar cells have the potential to achieve conversion efficiencies beyond the Shockley-Queisser (S-Q) limit while also significantly lowering production costs.
Protocols
Fabrication of Poly(triaryl amine) Field-effect Transistors
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![Poly[bis(4-phenyl)(2,4,6-trimethylphenyl)amine]](/deepweb/assets/sigmaaldrich/product/structures/288/293/16f2da62-4f58-4b77-926a-5bf2a96e0ad8/640/16f2da62-4f58-4b77-926a-5bf2a96e0ad8.png)
![[6,6]-Phenyl C61 butyric acid methyl ester ≥99%](/deepweb/assets/sigmaaldrich/product/structures/359/221/d990c746-0960-4c69-bf76-fe09b193824d/640/d990c746-0960-4c69-bf76-fe09b193824d.png)