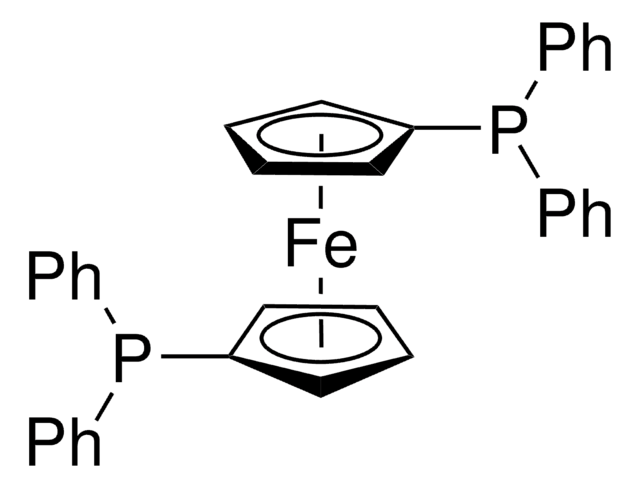
701998
[1,1′-Bis(di-cyclohexylphosphino)ferrocene]dichloropalladium(II)
98%
Synonym(s):
PdCl2(dcypf)
About This Item
Recommended Products
Quality Level
Assay
98%
form
powder
reaction suitability
core: palladium
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Cross Couplings
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: catalyst
mp
294-300 °C
storage temp.
−20°C
SMILES string
[Fe].Cl[Pd]Cl.[CH]1[CH][CH][C]([CH]1)P(C2CCCCC2)C3CCCCC3.[CH]4[CH][CH][C]([CH]4)P(C5CCCCC5)C6CCCCC6
InChI
1S/2C17H26P.2ClH.Fe.Pd/c2*1-3-9-15(10-4-1)18(17-13-7-8-14-17)16-11-5-2-6-12-16;;;;/h2*7-8,13-16H,1-6,9-12H2;2*1H;;/q;;;;;+2/p-2
InChI key
HRAMBTUEZQOQRK-UHFFFAOYSA-L
Application
- α, β-unsaturated amides by hydroaminocarbonylation of alkynes with tertiary amines.
- Quinazoline derivatives via Suzuki-Miyaura coupling reaction between of quinazolines containing unprotected NH2 group and arylboronic acids.
- α-aryl carbonyl compounds by α-arylation of ketones with aryl chlorides and aryl bromides.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service![[1,1′-Bis(di-tert-butylphosphino)ferrocene]dichloropalladium(II) 98%](/deepweb/assets/sigmaaldrich/product/structures/192/459/02d1239c-1119-49d9-b392-a04d8f53855c/640/02d1239c-1119-49d9-b392-a04d8f53855c.png)
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II), complex with dichloromethane](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)
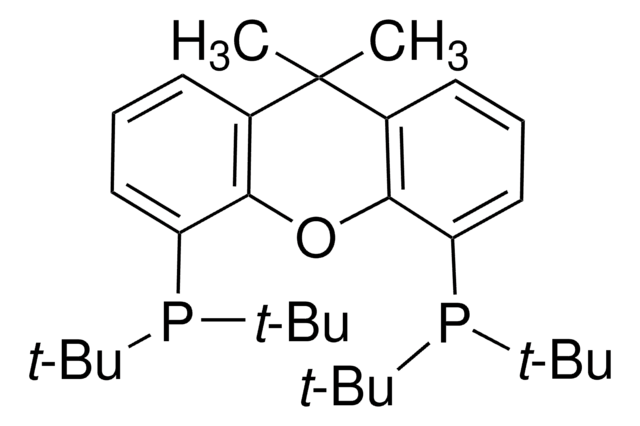
![Dichloro[9,9-dimethyl-4,5-bis(diphenylphosphino)xanthene]palladium(II) 95%](/deepweb/assets/sigmaaldrich/product/structures/374/597/f7932c5b-0448-498b-8254-f8ce1b9a4612/640/f7932c5b-0448-498b-8254-f8ce1b9a4612.png)
![Dichloro[bis(2-(diphenylphosphino)phenyl)ether]palladium(II) 95%](/deepweb/assets/sigmaaldrich/product/structures/311/408/0ee427f0-19c0-413a-8f38-827359ddbcac/640/0ee427f0-19c0-413a-8f38-827359ddbcac.png)
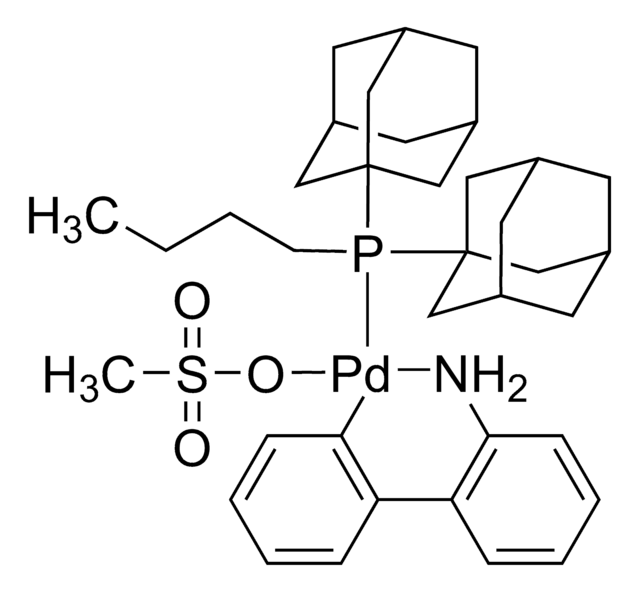
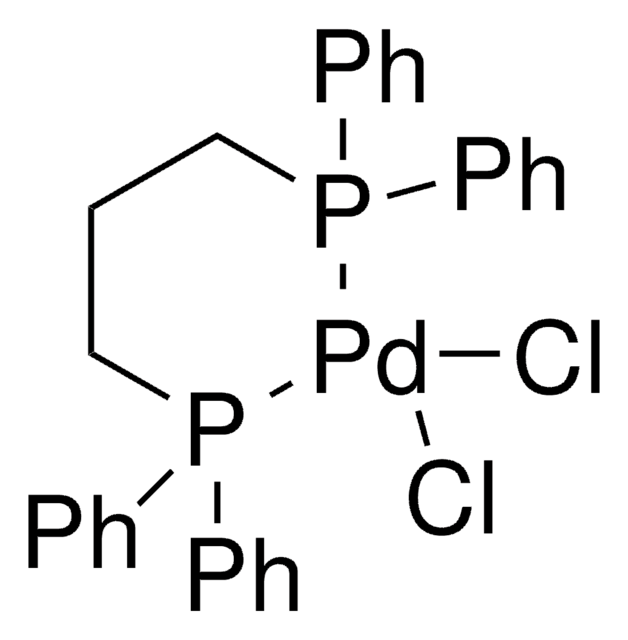
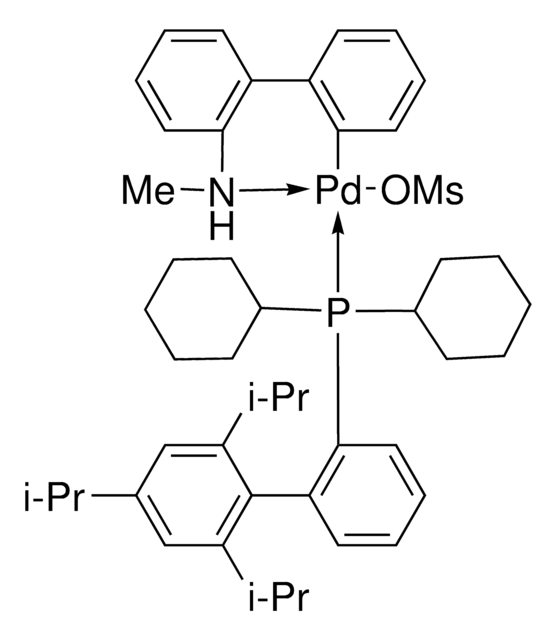

![(R)-1-[(SP)-2-(Dicyclohexylphosphino)ferrocenyl]ethyldi-tert-butylphosphine ≥97%](/deepweb/assets/sigmaaldrich/product/structures/809/974/e027b628-7c2e-4bde-be7e-f9298d0c8b04/640/e027b628-7c2e-4bde-be7e-f9298d0c8b04.png)