692786
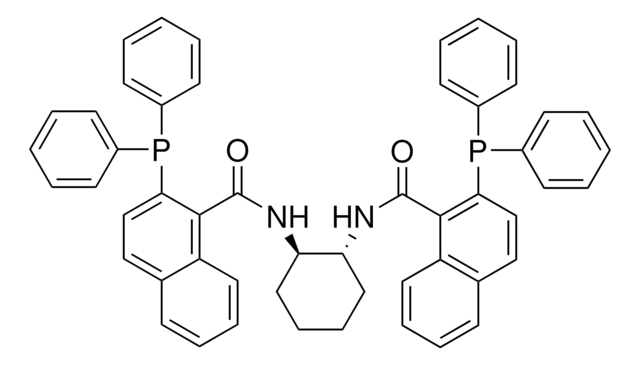
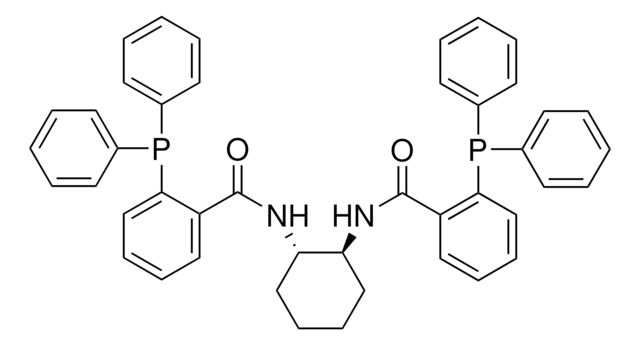
(S,S)-DACH-naphthyl Trost Ligand
95%
Synonym(s):
(1S,2S)-(–)-1,2-Diaminocyclohexane-N,N′-bis(2-diphenylphosphino-1-naphthoyl)
About This Item
Recommended Products
Quality Level
Assay
95%
form
solid
optical activity
[α]20/D -66.0°, c = 1 in methanol
mp
232-237 °C
SMILES string
O=C(N[C@H]1CCCC[C@@H]1NC(=O)c2c(ccc3ccccc23)P(c4ccccc4)c5ccccc5)c6c(ccc7ccccc67)P(c8ccccc8)c9ccccc9
InChI
1S/C52H44N2O2P2/c55-51(49-43-29-15-13-19-37(43)33-35-47(49)57(39-21-5-1-6-22-39)40-23-7-2-8-24-40)53-45-31-17-18-32-46(45)54-52(56)50-44-30-16-14-20-38(44)34-36-48(50)58(41-25-9-3-10-26-41)42-27-11-4-12-28-42/h1-16,19-30,33-36,45-46H,17-18,31-32H2,(H,53,55)(H,54,56)/t45-,46-/m0/s1
InChI key
VXFKMKXTPXVEMU-ZYBCLOSLSA-N
Related Categories
Legal Information
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Palladium-catalyzed asymmetric allylic alkylation (AAA) has proven to be an exceptionally powerful method for the efficient construction of stereogenic centers.
The Trost group at Stanford University has pioneered the use of C-2 symmetric diaminocyclohexyl (DACH) ligands in AAA, allowing for the rapid synthesis of a diverse range of chiral products with a limited number of chemical transformations.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service