All Photos(3)
About This Item
Empirical Formula (Hill Notation):
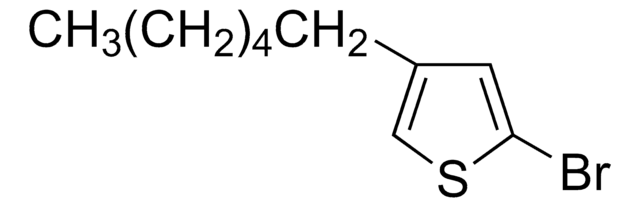
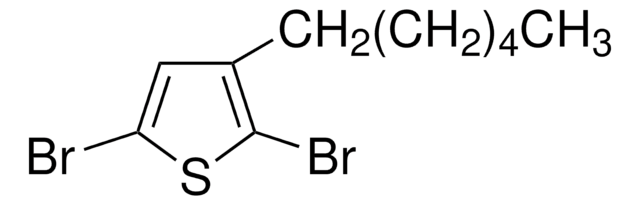
C10H15BrS
CAS Number:
Molecular Weight:
247.20
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Assay
97%
form
liquid
refractive index
n20/D 1.529
density
1.240 g/mL at 25 °C
SMILES string
CCCCCCc1ccsc1Br
InChI
1S/C10H15BrS/c1-2-3-4-5-6-9-7-8-12-10(9)11/h7-8H,2-6H2,1H3
InChI key
XQJNXCHDODCAJF-UHFFFAOYSA-N
General description
2-Bromo-3-hexylthiophene is a monomeric precursor that forms bromo terminate polymers. It is synthesized by the bromination of hexylthiophene.
Application
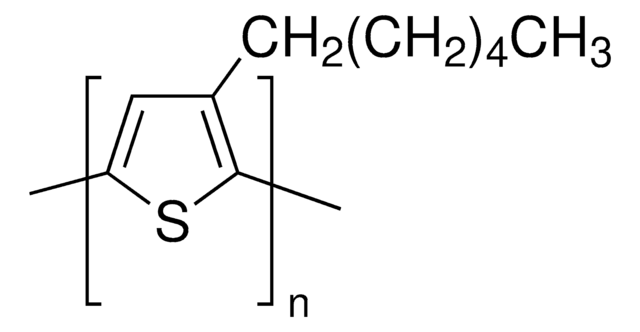
2-Bromo-3-hexylthiophene is majorly used in the formation of π-conjugated conductive polymers (CPs) for the fabrication of organic field effect transistors (OFETs) and organic photovoltaics (OPVs). The Grignard metathesis (GRIM) polymerization of 2-Bromo-3-hexylthiophene terminates a bromine and a proton at both ends to form conductive poly(3-hexylthiophene) (P3HT).
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral - Aquatic Chronic 4
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
>230.0 °F
Flash Point(C)
> 110 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Grignard metathesis (GRIM) polymerization for the synthesis of conjugated block copolymers containing regioregular poly (3-hexylthiophene).
Stefan MC, et al.
Polym. Chem., 3(7), 1693-1701 (2012)
Field-effect transistors based on poly (3-hexylthiophene): Effect of impurities.
Urien M, et al.
Organic Electronics, 8(6), 727-734 (2007)
Synthesis of End-capped Regioregular Poly (3-hexylthiophene) s via Direct Arylation.
Wang Q, et al.
Macromolecular Rapid Communications, 33(14), 1203-1207 (2012)
Synthesis and characterization of poly (3-hexylthiophene)-b-polystyrene for photovoltaic application.
Gu Z, et al.
Polymers, 3(1), 558-570 (2011)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)

![[1,3-Bis(diphenylphosphino)propane]dichloronickel(II)](/deepweb/assets/sigmaaldrich/product/structures/844/065/af07f787-c6a3-4a6e-a22b-47a933c73978/640/af07f787-c6a3-4a6e-a22b-47a933c73978.png)