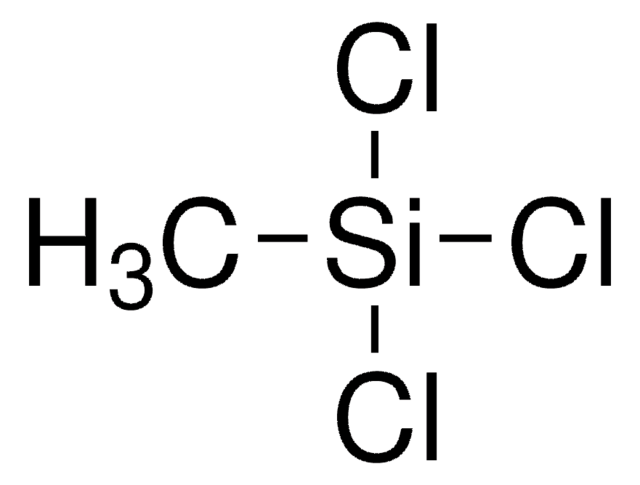679208
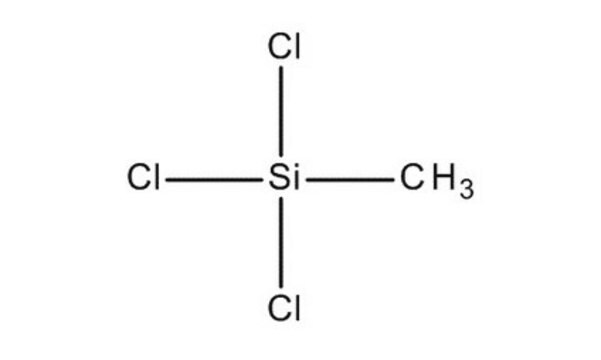
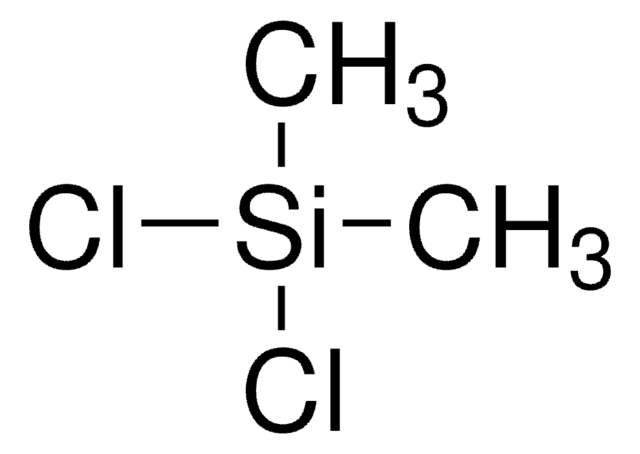
Methyltrichlorosilane
deposition grade, ≥98% (GC), ≥99.99% (as metals)
Synonym(s):
Trichloro(methyl)silane
About This Item
Recommended Products
grade
deposition grade
Quality Level
vapor density
5.2 (vs air)
vapor pressure
150 mmHg ( 25 °C)
Assay
≥98% (GC)
≥99.99% (as metals)
form
liquid
autoignition temp.
>760 °F
expl. lim.
11.9 %
refractive index
n20/D 1.411 (lit.)
bp
66 °C (lit.)
density
1.273 g/mL at 25 °C (lit.)
SMILES string
C[Si](Cl)(Cl)Cl
InChI
1S/CH3Cl3Si/c1-5(2,3)4/h1H3
InChI key
JLUFWMXJHAVVNN-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Inhalation - Acute Tox. 4 Dermal - Acute Tox. 4 Oral - Eye Dam. 1 - Flam. Liq. 2 - Skin Corr. 1A - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point(F)
46.4 °F - closed cup
Flash Point(C)
8 °C - closed cup
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Deposition Grade Silanes, fully characterized by chemical analysis and nuclear magnetic resonance (NMR) with greater than 98% purity, for Sol-Gel Processes.
Research involving reactive silicone chemistry has focused on the production of pure silicon and hybrid materials, hydrosilylation, ring-opening and atom transfer polymerizations, polymerizations with controlled stereochemistry, and condensation reactions.
atomic layer deposition (ALD), microelectronics, Mo:Al2O3 films, nanocomposite coating, photovoltaics, semiconductor devices, W:Al2O3 films, composite films, layer-by-layer
Silica is a very popular inorganic nanomaterial used in a wide range of applications including fillers for rubber, catalyst supports, separation media, carriers in food and agriculture, and abrasive/anticaking agents in cosmetics. It is also widely believed to be an important material for biomedical applications for following reasons.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service