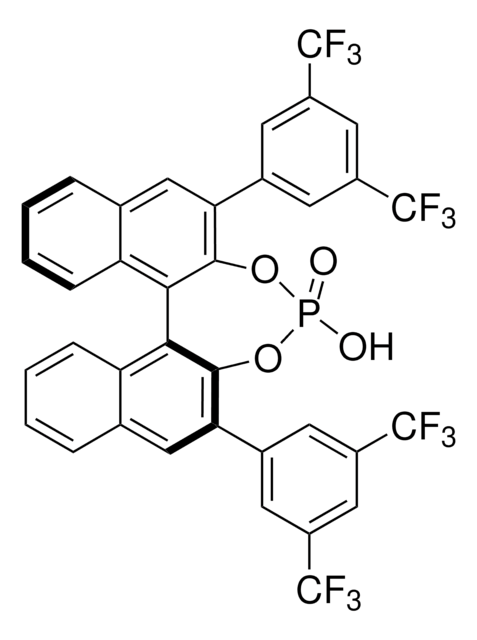
674591
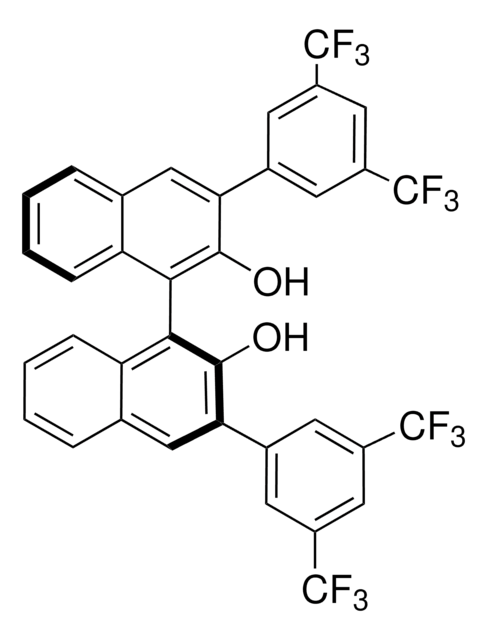
(R)-(+)-3,3′-Bis(3,5-bis(trifluoromethyl)phenyl)-1,1′-bi-2-naphthol
95%
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C36H18F12O2
CAS Number:
Molecular Weight:
710.51
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
form
solid
optical activity
[α]22/D 45°, c = 1 in chloroform
mp
216-220 °C
InChI
1S/C36H18F12O2/c37-33(38,39)21-9-19(10-22(15-21)34(40,41)42)27-13-17-5-1-3-7-25(17)29(31(27)49)30-26-8-4-2-6-18(26)14-28(32(30)50)20-11-23(35(43,44)45)16-24(12-20)36(46,47)48/h1-16,49-50H
InChI key
PGXMYJSTCAQJBY-UHFFFAOYSA-N
Application
Precursor to a chiral Bronsted acid (680184) used to catalyze an enantioselective aza Diels-Alder reaction providing bicyclic lactams. Rare earth metal complexes of this chiral binapthol catalyze an intramolecular hydroamination of amino olefins leading to chiral pyrrolidines.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
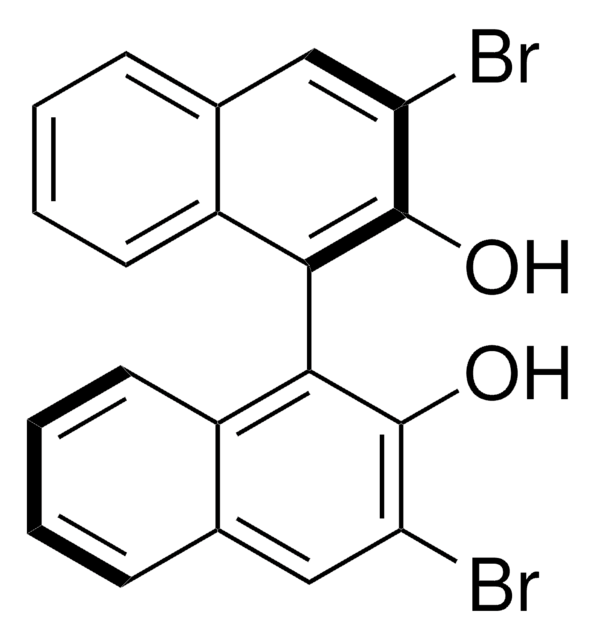
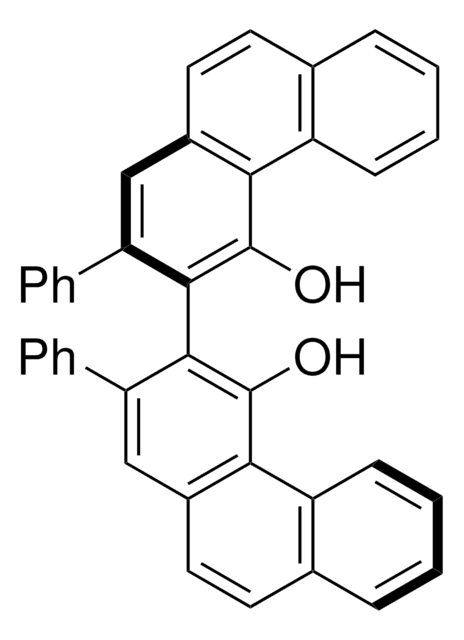
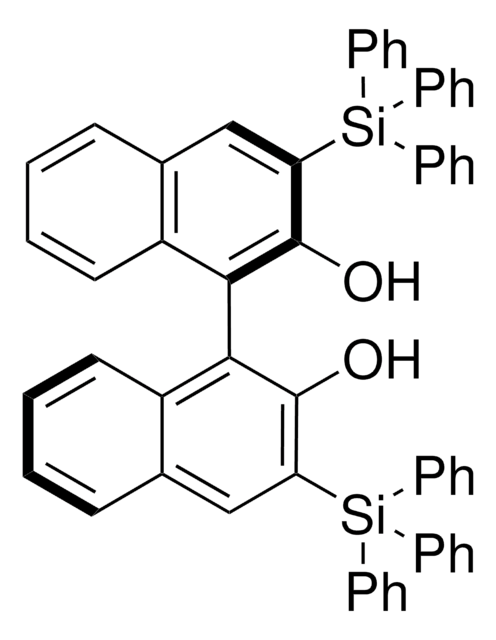
Customers Also Viewed
Hua Liu et al.
Organic letters, 8(26), 6023-6026 (2006-12-15)
[Structure: see text] The first chiral Brønsted acid-catalyzed asymmetric direct aza hetero-Diels-Alder reaction has been described. The phosphoric acids, prepared from binol and H8-binol derivatives, have shown catalytic ability for the reaction of cyclohexenone with N-PMP-benzaldimine. A chiral phosphoric acid
Denis V Gribkov et al.
Journal of the American Chemical Society, 128(11), 3748-3759 (2006-03-16)
Chiral 3,3'-bis(trisarylsilyl)-substituted binaphtholate rare earth metal complexes (R)-[Ln{Binol-SiAr3}(o-C6H4CH2NMe2)(Me2NCH2Ph)] (Ln = Sc, Lu, Y; Binol-SiAr3 = 3,3'-bis(trisarylsilyl)-2,2'-dihydroxy-1,1'-binaphthyl; Ar = Ph (2-Ln), 3,5-xylyl (3-Ln)) and (R)-[La{Binol-Si(3,5-xylyl)3}{E(SiMe3)2}(THF)2] (E = CH (4a), N (4b)) are accessible via facile arene, alkane, and amine elimination. They
Articles
We present an article concerning BINOL and Derivatives.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![(R)-3,3′-Bis[3,5-bis(trifluoromethyl)phenyl]-1,1′-binaphthyl-2,2′-diyl hydrogenphosphate 95%](/deepweb/assets/sigmaaldrich/product/structures/270/636/14dc9413-bcb4-478c-8e4d-3605317c13a5/640/14dc9413-bcb4-478c-8e4d-3605317c13a5.png)