666181
1,3-Dicyclohexylimidazolium tetrafluoroborate salt
97%
Synonym(s):
1,3-Bis(cyclohexyl)imidazolium tetrafluoroborate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C15H25BF4N2
CAS Number:
Molecular Weight:
320.18
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
solid
reaction suitability
reagent type: catalyst
mp
171-175 °C
SMILES string
F[B-](F)(F)F.C1CCC(CC1)n2cc[n+](c2)C3CCCCC3
InChI
1S/C15H25N2.BF4/c1-3-7-14(8-4-1)16-11-12-17(13-16)15-9-5-2-6-10-15;2-1(3,4)5/h11-15H,1-10H2;/q+1;-1
InChI key
CQHXJIHJFMBBQA-UHFFFAOYSA-N
Application
Used as organocatalyst in the catalytic boron conjugate additions to cyclic and acyclic unsaturated carbonyls
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Kang-sang Lee et al.
Journal of the American Chemical Society, 131(21), 7253-7255 (2009-05-13)
Metal-free nucleophilic activation of a B-B bond has been exploited in the development of a highly efficient method for conjugate additions of commercially available bis(pinacolato)diboron to cyclic or acyclic alpha,beta-unsaturated carbonyls. The reactions are readily catalyzed by a simple N-heterocyclic
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service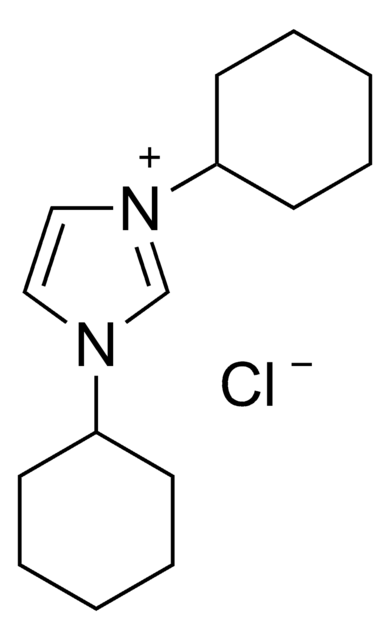








![Chloro[1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidene]copper(I)](/deepweb/assets/sigmaaldrich/product/structures/199/763/44637b2e-b87c-42a3-abc3-3985b6cd7d5d/640/44637b2e-b87c-42a3-abc3-3985b6cd7d5d.png)
![Chloro[1,3-Bis(2,4,6-trimethylphenyl)imidazol-2-ylidene]copper(I) 95%](/deepweb/assets/sigmaaldrich/product/structures/160/888/97509eeb-0719-4853-aaae-8a9d02f4f7ad/640/97509eeb-0719-4853-aaae-8a9d02f4f7ad.png)