665096
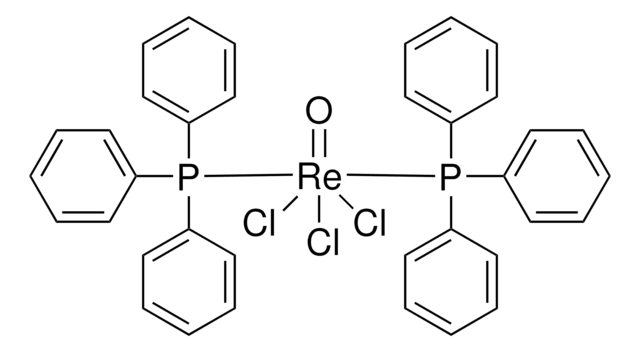
Oxotrichloro[(dimethylsulfide)triphenylphosphine oxide]rhenium(V)
97%
Synonym(s):
Trichlorooxo[thiobis[methane]](triphenylphosphine oxide-κO)rhenium
About This Item
Recommended Products
Quality Level
Assay
97%
form
powder
reaction suitability
core: rhenium
reagent type: catalyst
SMILES string
CSC.Cl[Re](Cl)(Cl)=O.O=P(c1ccccc1)(c2ccccc2)c3ccccc3
InChI
1S/C18H15OP.C2H6S.3ClH.O.Re/c19-20(16-10-4-1-5-11-16,17-12-6-2-7-13-17)18-14-8-3-9-15-18;1-3-2;;;;;/h1-15H;1-2H3;3*1H;;/q;;;;;;+3/p-3
InChI key
GIBDGLAUIKUJJX-UHFFFAOYSA-K
Application
- Enantioselective reduction of ketones and imines
- Meyer-Schuster rearrangement of propargylic alcohols to α, β-unsaturated carbonyl compounds
- Synthesis of 2-deoxy-α glycosides
- Sulfurization of phosphorous(III) compounds with thiiranes
Reactant for synthesis of mono and dinuclear compounds
Reagent for rhenium-cyclized somatostatine derivative series synthesis
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Rhenium(V) forms a large number of stable octahedral complexes with multiple bonds to oxygen with traditional Re systems focusing on formal, stoichiometric oxygen atom transfer to organic reductants such as phosphines, alkenes, and sulfides.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service