658405
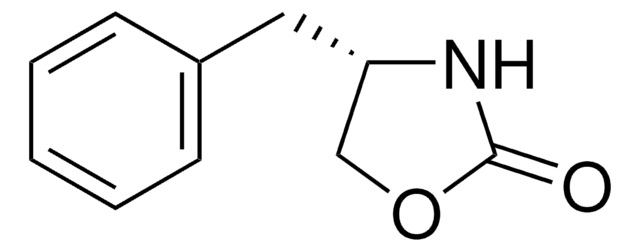
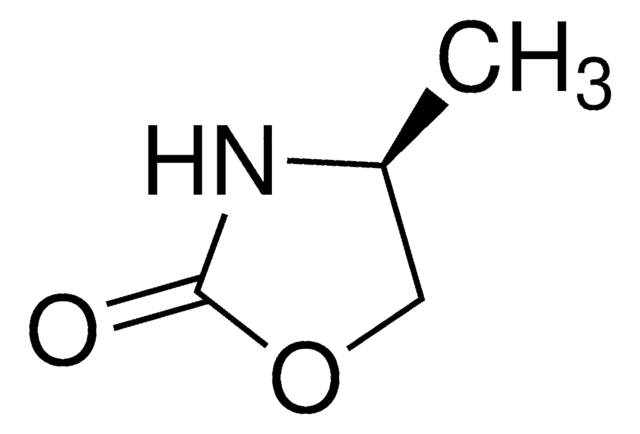
(S)-4-(4-Aminobenzyl)-2(1H)-oxazolidinone
97%
Synonym(s):
(4S)-4-[(4-Aminophenyl)methyl]-2-oxazolidinone
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C10H12N2O2
CAS Number:
Molecular Weight:
192.21
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
solid
mp
107-111 °C
SMILES string
Nc1ccc(C[C@H]2COC(=O)N2)cc1
InChI
1S/C10H12N2O2/c11-8-3-1-7(2-4-8)5-9-6-14-10(13)12-9/h1-4,9H,5-6,11H2,(H,12,13)/t9-/m0/s1
InChI key
WNAVSKJKDPLWBD-VIFPVBQESA-N
Looking for similar products? Visit Product Comparison Guide
Application
(S)-4-(4-Aminobenzyl)-2(1H)-oxazolidinone can be used as a starting material in the synthesis of:
- Oxazolidinone derived 2-azetidinones as metal cation sensors.
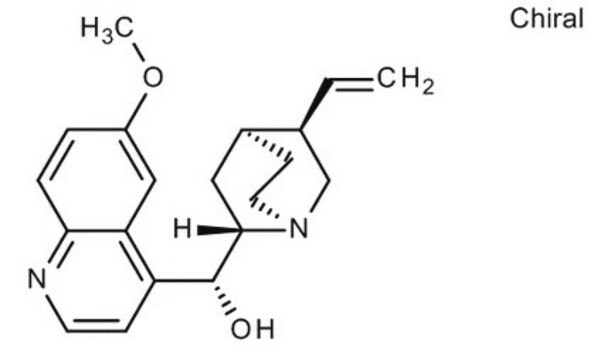
- Serotonin receptor agonist named zolmitriptan.
- Schiff base derived zinc metal complexes of biological importance.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
(S)-4-(4-aminobenzyl)-2-oxazolidinone based 2-azetidinones for antimicrobial application and luminescent sensing of divalent metal cations
Baruah S, et al.
Journal of Heterocyclic Chemistry, 57, 2498?2511-2498?2511 (2020)
Convenient and Industrially Viable Process for Preparation of Zolmitriptan
Neelakandan K, et al.
Journal of Heterocyclic Chemistry, 51(S1), E332-E334 (2014)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service