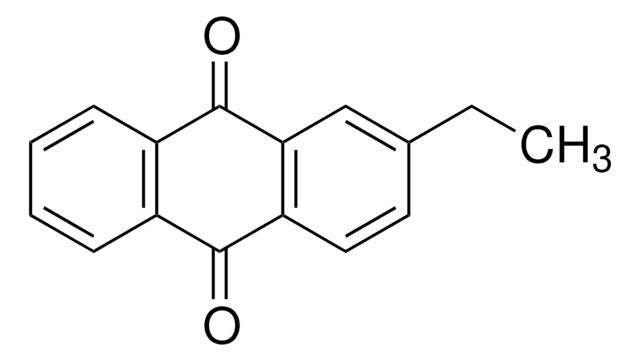
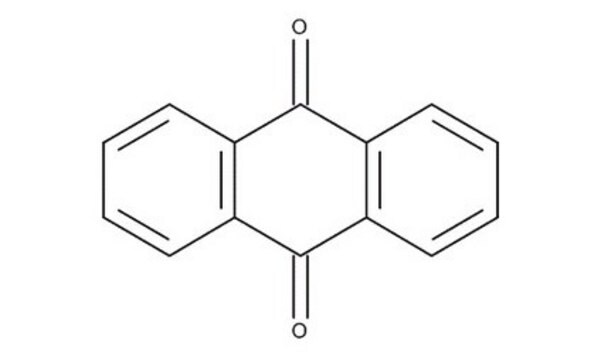
65800
2-Methylanthraquinone
technical, ≥95% (HPLC)
Synonym(s):
2-MAQ
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C15H10O2
CAS Number:
Molecular Weight:
222.24
Beilstein:
2050523
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
technical
Quality Level
Assay
≥95% (HPLC)
impurities
3-4% 1-methylanthraquinone
bp
236-238 °C/10 mmHg (lit.)
mp
170-173 °C (lit.)
SMILES string
Cc1ccc2C(=O)c3ccccc3C(=O)c2c1
InChI
1S/C15H10O2/c1-9-6-7-12-13(8-9)15(17)11-5-3-2-4-10(11)14(12)16/h2-8H,1H3
InChI key
NJWGQARXZDRHCD-UHFFFAOYSA-N
Gene Information
human ... CTSG(1511) , ELA2(1991)
Looking for similar products? Visit Product Comparison Guide
Application
2-Methylanthraquinone may be used in the preparation of potential bioreducible anthraquinone derivatives.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
408.2 °F - closed cup
Flash Point(C)
209 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Giampaolo Gori et al.
The Annals of occupational hygiene, 53(1), 27-32 (2008-11-04)
A new gas chromatographic/mass spectrometric (GC/MS) method was developed to detect 2-methylanthraquinone (2-MeA) in wood dust. 2-MeA is present in teak wood (a suspected human carcinogen) but not in oak, beech, mahogany, birch, ash or pine. The method involved collection
Effect of additives on fiber yield improvement for kraft pulping of kadam (Anthocephalus chinensis).
D Biswas et al.
Bioresource technology, 102(2), 1284-1288 (2010-09-11)
Projected decline in future wood resources has prompted researchers to try various additives in existing pulping processes for fiber yield improvement. Many studies have been conducted in the past aimed at improving kraft pulp yield with the use of additives
Emily L Whitson et al.
Journal of natural products, 75(3), 394-399 (2012-02-09)
Barleria alluaudii and Diospyros maritima were both investigated as part of an ongoing search for synergistic TRAIL (tumor necrosis factor-α-related apoptosis-inducing ligand) sensitizers. As a result of this study, two naphthoquinone epoxides, 2,3-epoxy-2,3-dihydrolapachol (1) and 2,3-epoxy-2,3-dihydro-8-hydroxylapachol (2), both not previously
Helen Sheridan et al.
Journal of natural products, 74(1), 82-85 (2010-12-23)
Two new furanonaphthoquinones, (3R)-7-methoxy-α-dunnione (5) and (3R)-6-hydroxy-7-methoxy-α-dunnione (6), along with the known (3R)-dunnione (1), (3R)-α-dunnione (2), (3R)-7-hydroxy-α-dunnione (3), and 1-hydroxy-2-methylanthraquinone (4), were isolated from in vitro cultures of Streptocarpus dunnii. The structures of compounds 5 and 6 were established by
Maria Helena Verdan et al.
Journal of natural products, 73(8), 1434-1437 (2010-08-06)
Three new aromatic epsilon-lactones, aggregatins A (1), B (2), and C (3), a new naphthoquinone derivative, aggregatin D (4), and three known anthraquinones, 2-methylanthraquinone, 7-methoxy-2-methylanthraquinone, and 7-hydroxy-2-methylanthraquinone, were isolated from the tubers of Sinningia aggregata (Gesneriaceae). Compounds 1 and 4
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service