All Photos(2)
About This Item
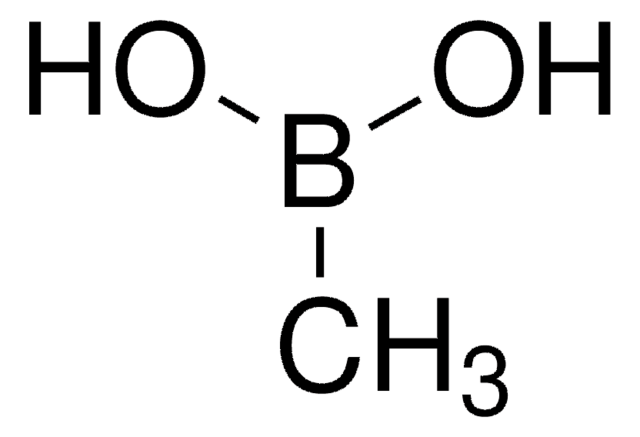
Empirical Formula (Hill Notation):
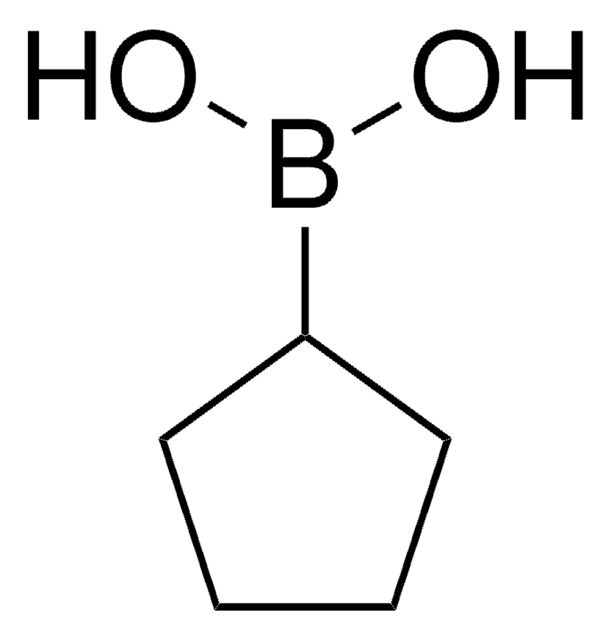
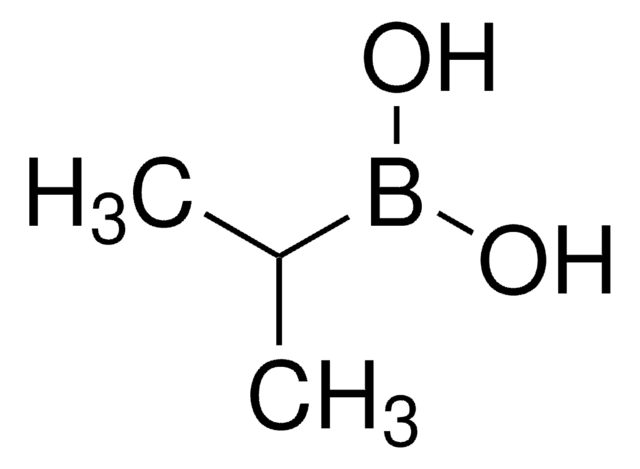
C4H9BO2
CAS Number:
Molecular Weight:
99.92
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥95.0%
form
solid
mp
118-123 °C (lit.)
storage temp.
2-8°C
SMILES string
OB(O)C1CCC1
InChI
1S/C4H9BO2/c6-5(7)4-2-1-3-4/h4,6-7H,1-3H2
InChI key
MIUALDDWOKMYDA-UHFFFAOYSA-N
Application
- Reagent used in palladium-catalyzed arylation and alkylation of diphenylisoxazole with boronic acids via C-H activated isoxazole palladacycle intermediate
- Reagent used in the preparation of cyclobutyl arenes and heteroarenes via palladium catalyzed Suzuki-Miyaura cross-coupling reaction of potassium cyclobutyltrifluoroborate intermediate with aryl and heteroaryl chlorides
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Palladium-Catalyzed Arylation and Alkylation of 3,5-Diphenylisoxazole with Boronic Acids via C-H Activation
J. Chu, et al.,
Organometallics, 27, 5173-5176 (2008)
Gary A Molander et al.
The Journal of organic chemistry, 73(19), 7481-7485 (2008-09-02)
Suitable conditions were found for the Suzuki-Miyaura cross-coupling reaction of potassium cyclopropyl- and cyclobutyltrifluoroborates with aryl chlorides. Both of these secondary alkyl trifluoroborates coupled in moderate to excellent yield with electron-rich, electron-poor, and hindered aryl chlorides to give a variety
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service