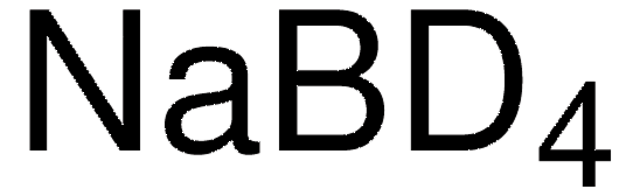632287
VenPure® SF
powder
Synonym(s):
Sodium borohydride, Sodium tetrahydridoborate
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
NaBH4
CAS Number:
Molecular Weight:
37.83
EC Number:
MDL number:
UNSPSC Code:
26111700
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
form
powder
Quality Level
reaction suitability
reagent type: reductant
mp
>300 °C (dec.) (lit.)
SMILES string
[BH4-].[Na+]
InChI
1S/BH4.Na/h1H4;/q-1;+1
InChI key
YOQDYZUWIQVZSF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
VenPure® SF (Sodium borohydride powder) is a mild reducing agent used in the reduction of aldehydes and ketones to their corresponding alcohols. It is generally inert to other functional groups such as epoxides, esters, nitriles and lactones. Along with carboxylic acid, it forms acyloxyborohydride, which is a versatile reducing agent to reduce indoles, quinolones, imines, enamines, oximes and enamides.
Legal Information
VenPure is a registered trademark of Ascensus Specialties LLC
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral - Eye Dam. 1 - Repr. 1B - Skin Corr. 1B - Water-react 1
Supplementary Hazards
Storage Class Code
4.3 - Hazardous materials which set free flammable gases upon contact with water
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Sodium borohydride in carboxylic acid media: a phenomenal reduction system.
Gribble G W
Chemical Society Reviews, 27(6), 395-404 (1998)
Sodium borohydride.
Banfi L, et al.
e-EROS Encyclopedia of Reagents for Organic Synthesis., 1-13 (2014)
Forty years of hydride reductions.
Brown H C and Krishnamurthy S
Tetrahedron, 35(5), 567-607 (1979)
Aldwin Suryo Rahmanto et al.
Free radical biology & medicine, 53(6), 1308-1316 (2012-08-14)
Singlet oxygen ((1)O(2)) is a reactive oxygen species generated during photo-oxidation, inflammation, and via peroxidase-catalyzed reactions (e.g., myeloperoxidase and eosinophil peroxidase). (1)O(2) oxidizes the free amino acids Trp, Tyr, His, Cys, and Met, and those species present on peptides/proteins, with
Meganne L Christian et al.
ACS nano, 6(9), 7739-7751 (2012-08-10)
Owing to its high storage capacity (10.8 mass %), sodium borohydride (NaBH(4)) is a promising hydrogen storage material. However, the temperature for hydrogen release is high (>500 °C), and reversibility of the release is unachievable under reasonable conditions. Herein, we
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service