About This Item
Recommended Products
Quality Level
Assay
97%
form
solid
mp
64-67 °C (lit.)
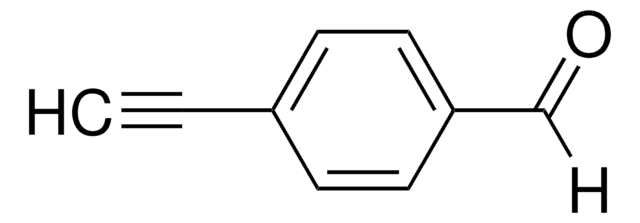
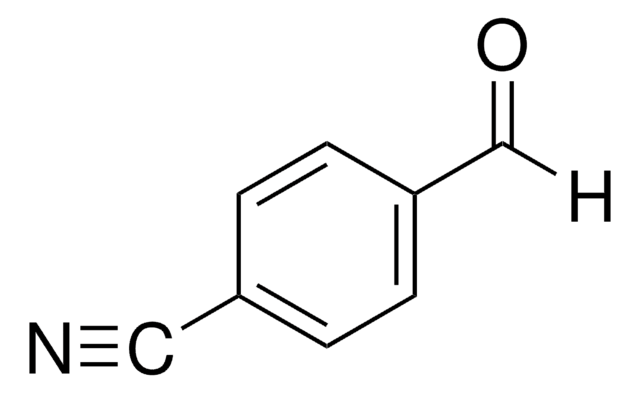
SMILES string
[H]C(=O)c1ccccc1C#C
InChI
1S/C9H6O/c1-2-8-5-3-4-6-9(8)7-10/h1,3-7H
InChI key
ZEDSAJWVTKUHHK-UHFFFAOYSA-N
Application
- iodoisoquinoline-fused benzimidazoles obtained via tandem iodocyclization of 2-ethynylbenzaldehyde with o-benzenediamine and iodine in the presence of copper(I)iodide
- N-[(7,7a-dihydroisoquinolino[2,1-a]perimidin-13-yl)methyl]-N-isopropylpropan-2-amine obtained via a multi-step process
- 2-[(2-bromophenyl)ethynyl]benzaldehyde
- 2-[(2-bromo-5-fluorophenyl)ethynyl]benzaldehyde
- 2-[(2-bromo-5-methylphenyl)ethynyl]benzaldehyde
- 2-(phenylethynyl)benzaldehyde
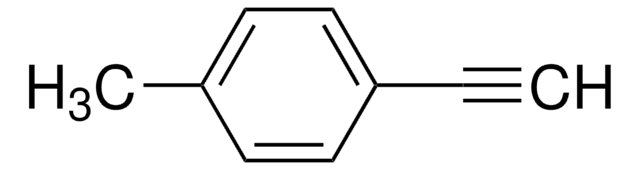
- 2-[(4-methylphenyl)ethynyl]benzaldehyde
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Iodoisoquinoline-Fused Benzimidazoles?
hydroamination of 2-ethynylbenzaldehydes?
Articles
The terminal alkyne functionality has a wide range of applications including most recently the synthesis of spiropyran substituted 2,3-dicyanopyrazines and (±)-asteriscanolide, as well as conversion to enamines using resin-bound 2° amines.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service