561673
1-Naphthylmagnesium bromide solution
0.25 M slurry in THF
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C10H7BrMg
CAS Number:
Molecular Weight:
231.37
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
reaction suitability
reaction type: Grignard Reaction
Quality Level
concentration
0.25 M slurry in THF
bp
65 °C
density
0.908 g/mL at 25 °C
SMILES string
Br[Mg]c1cccc2ccccc12
InChI
1S/C10H7.BrH.Mg/c1-2-6-10-8-4-3-7-9(10)5-1;;/h1-7H;1H;/q;;+1/p-1
InChI key
PZIIGUMPOSVMSD-UHFFFAOYSA-M
Application
1-Naphthylmagnesium bromide can be used:
- To prepare unsymmetrical chiral diene ligands, which are applicable in asymmetric transformation reactions.
- As a starting material in the synthesis of methyl (2Z,4E)-2-methylsulfanyl-5-(1-naphthyl)-4-nitro-2,4-pentadienoate, a naphthylnitrobutadiene based anti-proliferative compound.
Legal Information
Product of Rieke Metals, Inc.
Rieke is a registered trademark of Rieke Metals, Inc.
Rieke is a registered trademark of Rieke Metals, Inc.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Carc. 2 - Flam. Liq. 2 - Skin Corr. 1B - STOT SE 3
Target Organs
Respiratory system
Supplementary Hazards
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
1.4 °F - closed cup
Flash Point(C)
-17 °C - closed cup
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Design, synthesis, and in vitro evaluation of new naphthylnitrobutadienes with potential antiproliferative activity: Toward a structure-activity correlation
Petrillo G, et al.
Bioorganic & Medicinal Chemistry, 16(1), 240-247 (2008)
Stefan Abele et al.
The Journal of organic chemistry, 77(10), 4765-4773 (2012-05-04)
An operationally simple and scalable synthesis of enantiomerically pure bicyclo[2.2.2]octadiene (bod*) ligands relying on an organocatalytic one-pot Michael addition-aldol reaction with cheap 2-cyclohexenone and phenylacetaldehyde is presented. The crystalline bicyclic product 4a (6-hydroxy-5-phenylbicyclo[2.2.2]octan-2-one) is transformed into phenylbicyclo[2.2.2]oct-5-en-2-one 2, a versatile
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service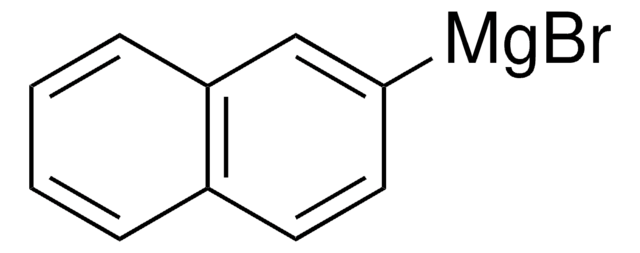

![4-[Bis(trimethylsilyl)amino]phenylmagnesium bromide solution 0.5 M in THF](/deepweb/assets/sigmaaldrich/product/structures/109/860/38618a54-089d-4f50-aacc-61f10c5c12ba/640/38618a54-089d-4f50-aacc-61f10c5c12ba.png)



