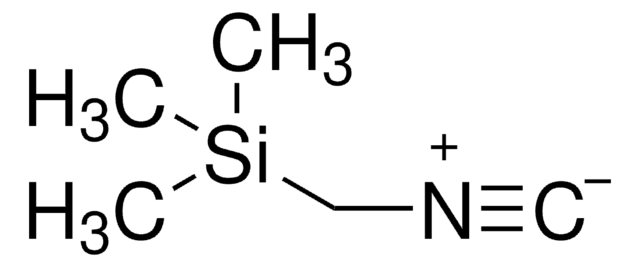553344
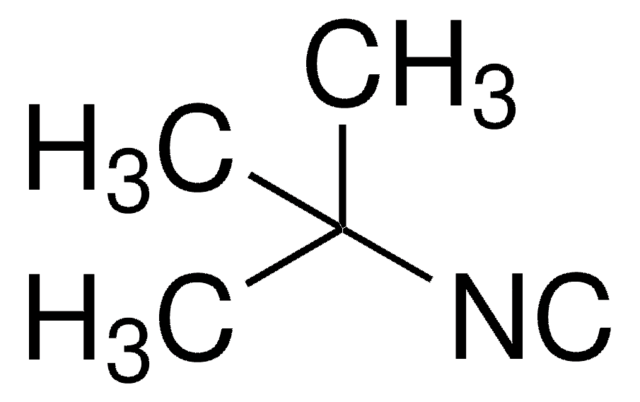
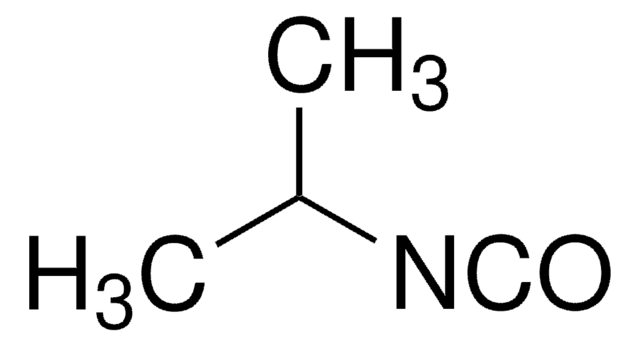
Isopropyl isocyanide
97%
Synonym(s):
2-Isocyanopropane, 2-Propyl isonitrile, Isopropyl isonitrile
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
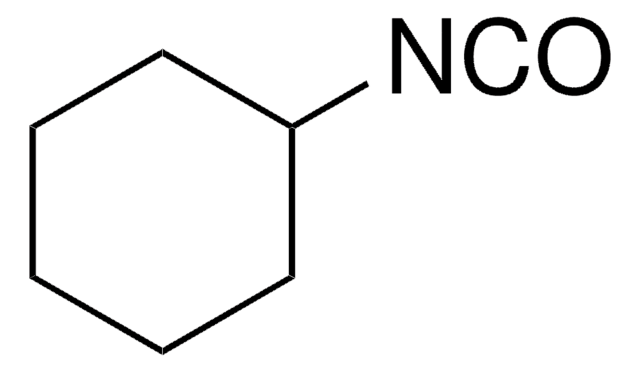
Linear Formula:
(CH3)2CHNC
CAS Number:
Molecular Weight:
69.11
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
refractive index
n20/D 1.371 (lit.)
bp
75 °C (lit.)
density
0.733 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
CC(C)[N+]#[C-]
InChI
1S/C4H7N/c1-4(2)5-3/h4H,1-2H3
InChI key
MJZUMMKYWBNKIP-UHFFFAOYSA-N
General description
Isopropyl isocyanide is an alkyl isocyanide.
Application
Isopropyl isocyanide may be used in the preparation of:
- Ugi ligand A2C11I1, which is employed in the solid-phase Ugi synthesis.
- Pentafluorophenyl (PFP) functional monomers, via Passerini reaction.
- Dinuclear copper complex [Cu2(μ-PiPr2bipy)2{μ-CNCH(CH3)2}](PF6)2 [PiPr2bipy = 6-(diisopropylphosphanyl)-2,2′-bipyridine].
- (3E)-(Imino)thiaisoindoline 1,1-dioxide derivative.
- N,N′-diisopropylcarbodiimide (DIC) analog via reaction with propan-2-amine.
- 1-t-butyl-2-isopropyldiaziridinone via reaction with 2-methyl-2-nitrosopropane
- isopropyl isocyanate via reaction with ozone
- 1-phthalimidoazetidines via [1+3]cycloaddition reaction with 1-pththalimidoaziridines
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
69.1 °F - closed cup
Flash Point(C)
20.6 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
[1+ 3] Cycloadditions of isocyanides to azomethine ylides; synthesis and properties of 1-phthalimidoazetidines
Charrier J, et al.
The Journal of Organic Chemistry, 48(4), 481-486 (1983)
Thiol-reactive functional poly (meth) acrylates: multicomponent monomer synthesis, RAFT (co) polymerization and highly efficient thiol-para-fluoro postpolymerization modification.
Noy JM, et al.
Polym. Chem., 6(3), 436-447 (2015)
Diaziridinones. IV. Formation by condensation of alkyl isocyanide with nitrosoalkane. Evidence for a carbodiimide N-oxide
Greene DF and Pazos FJ
The Journal of Organic Chemistry, 34(8), 2269-2274 (1969)
Synthesis of Carbodiimides by I2/CHP-Mediated Cross-Coupling Reaction of Isocyanides with Amines under Metal-Free Conditions.
Zhu TH, et al.
Organic Letters, 17(8), 1974-1977 (2015)
F C Rhames et al.
Journal of biological inorganic chemistry : JBIC : a publication of the Society of Biological Inorganic Chemistry, 6(5-6), 567-577 (2001-07-27)
Binding of the Cu(I)-specific ligands 2,6-dimethylphenyl isocyanide (DIMPI) and isopropyl isocyanide (IPI) to the reduced form of peptidylglycine monooxygenase (PHM) is reported. Both ligands bind to the methionine-containing CuM center, eliciting FTIR bands at 2,138 and 2,174 cm(-1), respectively, but
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service