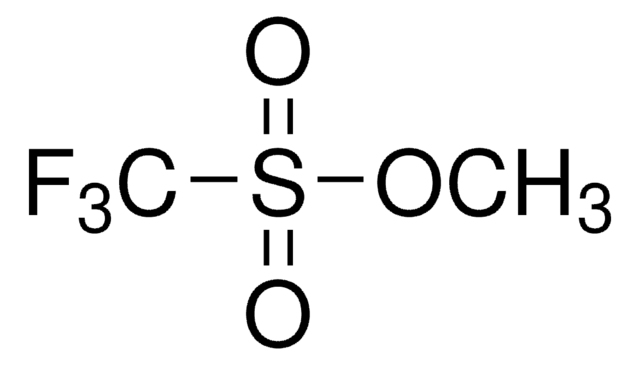
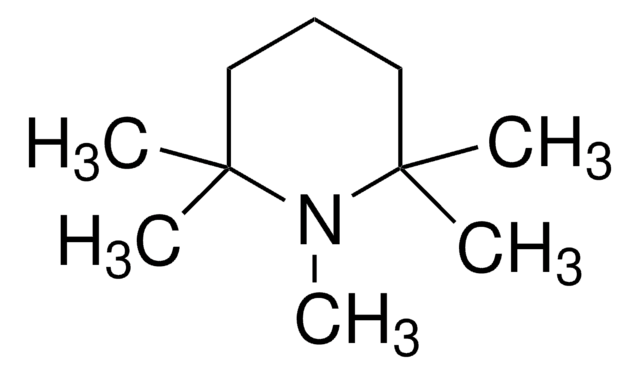
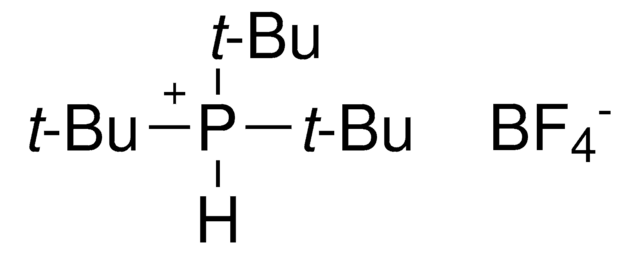549967
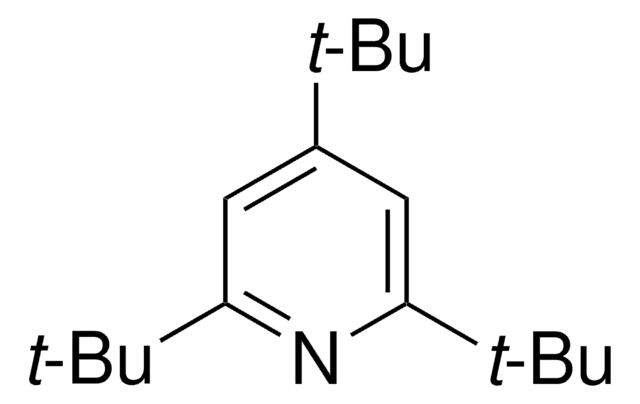
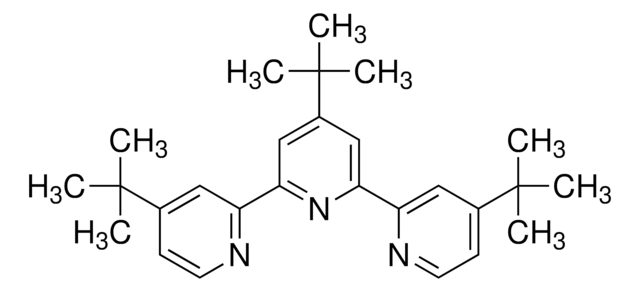
2,4,6-Tri-tert-butylpyrimidine
97%
Synonym(s):
2,4,6-Tris(1,1-dimethylethyl)pyrimidine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C16H28N2
CAS Number:
Molecular Weight:
248.41
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
mp
77-80 °C (lit.)
SMILES string
CC(C)(C)c1cc(nc(n1)C(C)(C)C)C(C)(C)C
InChI
1S/C16H28N2/c1-14(2,3)11-10-12(15(4,5)6)18-13(17-11)16(7,8)9/h10H,1-9H3
InChI key
VYWSYEDVFVGRGG-UHFFFAOYSA-N
General description
2,4,6-Tri-tert-butylpyrimidine (TTBP) is a non-hygroscopic sterically hindered base. It can be prepared by the reaction between tert-butyl methyl ketone and tert-butyronitrile.3 TTBP is a suitable alternative to 2,6-di-tert-butylated pyridines in glycosylation reactions.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
1-Benzenesulfinyl piperidine/trifluoromethanesulfonic anhydride: a potent combination of shelf-stable reagents for the low-temperature conversion of thioglycosides to glycosyl triflates and for the formation of diverse glycosidic linkages.
Crich D and Smith M.
Journal of the American Chemical Society, 123(37), 9015-9020 (2001)
2, 4, 6-Tri-tert-butylpyrimidine (TTBP): A cost effective, readily available alternative to the hindered base 2, 6-di-tert-butylpyridine and its 4-substituted derivatives in glycosylation and other reactions.
Crich D, et al.
Synthesis, 2001(02), 0323-0326 (2001)
On the mechanism of the reaction between ketones and trifluoromethanesulfonic anhydride. An improved and convenient method for the preparation of pyrimidines and condensed pyrimidines.
Garcia Martinez A, et al.
The Journal of Organic Chemistry, 57(5), 1627-1630 (1992)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service




![[1,3-Bis(2,6-diisopropylphenyl)imidazol-2-ylidene] [bis(trifluoromethanesulfonyl)imide]gold(I) 95%](/deepweb/assets/sigmaaldrich/product/structures/336/250/c96c15b3-1a1c-479f-a588-76e98905be23/640/c96c15b3-1a1c-479f-a588-76e98905be23.png)