All Photos(1)
About This Item
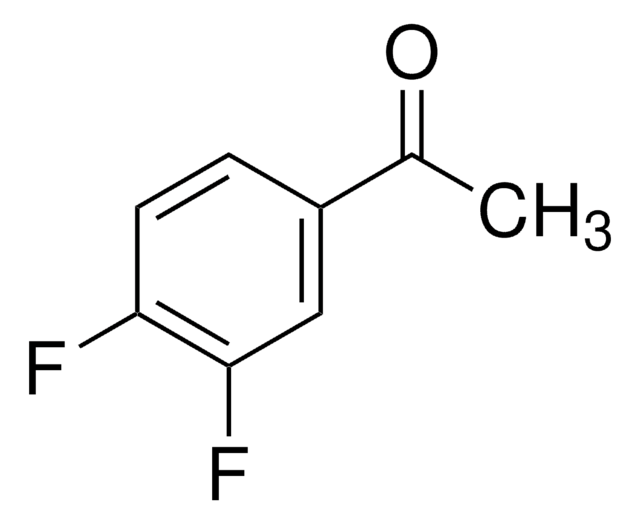
Linear Formula:
F2C6H3COCH3
CAS Number:
Molecular Weight:
156.13
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
mp
34-38 °C (lit.)
SMILES string
CC(=O)c1cc(F)cc(F)c1
InChI
1S/C8H6F2O/c1-5(11)6-2-7(9)4-8(10)3-6/h2-4H,1H3
InChI key
OXJLDNSPGPBDCP-UHFFFAOYSA-N
General description
3′,5′-Difluoroacetophenone, also known as 1-(3,5-difluorophenyl)ethanone, is a fluorinated acetophenone.
Application
3′,5′-Difluoroacetophenone (3,5-Difluoroacetophenone) may be used to synthesize:
- (E)-3-(4-(1,5,9-trithia-13-azacyclohexadecan-13-yl)-phenyl)-1-(3,5-difluorophenyl)prop-2-en-1-one
- 1,3,5-triarylpyrazoline fluorophores containing a 16-membered thiazacrown ligand
- (±)-fluorinated-1-(3-morpholin-4-yl-phenyl)ethylamine
- (E)-1-(3,5-difluorophenyl)-3-(2,4-dimethoxyphenyl) prop-2-en-1-one
- 1-(3,5-difluorophenyl)-4,4,4-trifluorobutane-1,3-dione
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
180.0 °F - closed cup
Flash Point(C)
82.2 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Yong-Jin Wu et al.
Bioorganic & medicinal chemistry letters, 13(10), 1725-1728 (2003-05-06)
The synthesis of four (+/-)-fluorinated 1-(3-morpholin-4-yl-phenyl)-ethylamines and an enantioselective approach to these amines through reductive amination are described.
Manjusha Verma et al.
Organic & biomolecular chemistry, 8(2), 363-370 (2010-01-13)
We have prepared and characterized a Cu(i)-responsive fluorescent probe, constructed using a large tetradentate, 16-membered thiazacrown ligand ([16]aneNS(3)) and 1,3,5-triaryl-substituted pyrazoline fluorophores. The fluorescence contrast ratio upon analyte binding, which is mainly governed by changes of the photoinduced electron transfer
1-(3, 5-Difluorophenyl)-4, 4, 4-trifluorobutane-1, 3-dione.
Manoj Kumar KE, et al.
Acta Crystallographica Section E, Structure Reports Online, 69(11), o1705-o1705 (2013)
(E)-1-(3, 5-Difluorophenyl)-3-(2, 4-dimethoxyphenyl) prop-2-en-1-one.
Huang T, et al.
Acta Crystallographica Section E, Structure Reports Online, 66(10), o2518-o2518 (2010)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service