540838
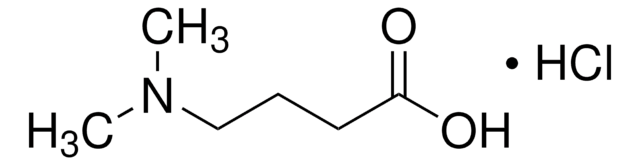
4-Piperidine butyric acid hydrochloride
97%
Synonym(s):
4-(Piperidin-4-yl)butanoic acid hydrochloride
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C9H18ClNO2
CAS Number:
Molecular Weight:
207.70
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
mp
113-117 °C (lit.)
SMILES string
Cl[H].OC(=O)CCCC1CCNCC1
InChI
1S/C9H17NO2.ClH/c11-9(12)3-1-2-8-4-6-10-7-5-8;/h8,10H,1-7H2,(H,11,12);1H
InChI key
UTPNREIRALGKPW-UHFFFAOYSA-N
Related Categories
General description
4-Piperidine butyric acid hydrochloride participates in the synthesis of FK866 [(E)-N-[4-(1-benzoylpiperidin-4-yl)butyl]-3-(pyridin-3-yl)acrylamide], an inhibitor of NAD biosynthesis.
Application
Reactant for:
Modification of 3-amidinophenylalanine-derived matriptase inhibitors
Reactions between Weinreb amides and 2-magnesiated oxazoles
Reactant for synthesis of:
NAmPRTase inhibitors
FK866 analogs for NAD salvage inhibition
Modification of 3-amidinophenylalanine-derived matriptase inhibitors
Reactions between Weinreb amides and 2-magnesiated oxazoles
Reactant for synthesis of:
NAmPRTase inhibitors
FK866 analogs for NAD salvage inhibition
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
210.2 °F - closed cup
Flash Point(C)
99 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Characterization of NAD uptake in mammalian cells.
Billington RA, et al.
The Journal of Biological Chemistry, 283(10), 6367-6374 (2008)
Ubaldina Galli et al.
ChemMedChem, 3(5), 771-779 (2008-02-06)
One of the great challenges of medicinal chemistry is to create novel, effective, chemotherapeutic agents that show specificity for cancer cells combined with low systemic toxicity. A novel idea is to target the enzymes of the NAD biosynthesis and recycling
Hyun You et al.
European journal of medicinal chemistry, 46(4), 1153-1164 (2011-02-19)
NAmPRTase (PBEF/Visfatin) plays a pivotal role in the salvage pathway of NAD(+) biosynthesis. NAmPRTase has been an attractive target for anti-cancer agents that induce apoptosis of tumor cells via a declining plasma NAD(+) level. In this report, a series of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service