All Photos(1)
About This Item
Linear Formula:
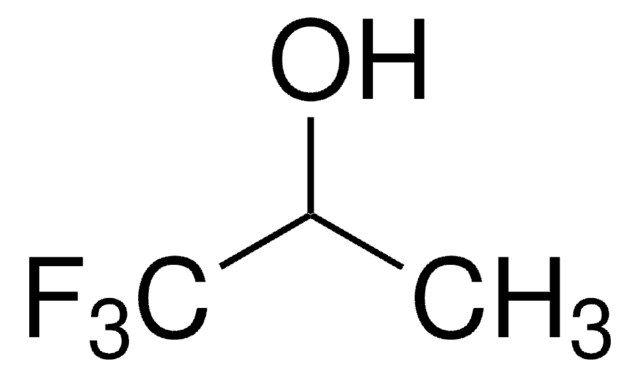
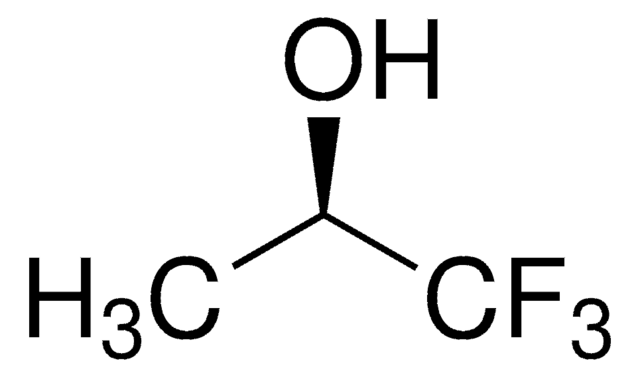
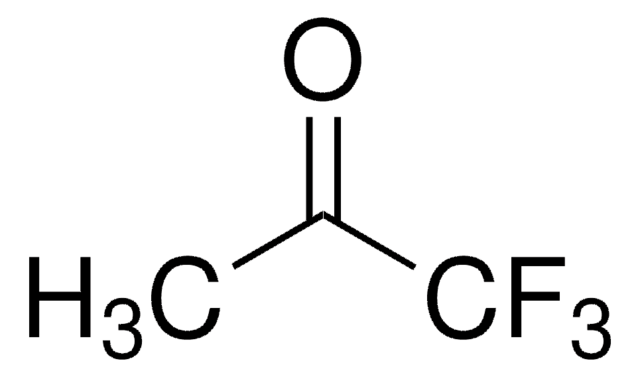
CF3C(CH3)2OH
CAS Number:
Molecular Weight:
128.09
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
96%
refractive index
n20/D 1.3335 (lit.)
bp
83 °C (lit.)
density
1.17 g/mL at 25 °C (lit.)
SMILES string
CC(C)(O)C(F)(F)F
InChI
1S/C4H7F3O/c1-3(2,8)4(5,6)7/h8H,1-2H3
InChI key
OCGWWLDZAFOHGD-UHFFFAOYSA-N
Related Categories
General description
2-Trifluoromethyl-2-propanol is a fluorinated aliphatic alcohol. It can be prepared by reacting methyllithium, trifluoroacetone and trifluorovinyl bromide in ether. This can also be synthesized from the reaction between methylmagnesium bromide and 1,1,1-trifluoroacetone in ether.
Application
2-Trifluoromethyl-2-propanol may be employed as a solvent for the preparation of [18F]fluorothymidine. It may also be used in the synthesis of [2-(trifluoromethyl)-2-propyl nitrate] through nitration in nitric acid/trifluoroacetic anhydride.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
59.0 °F - closed cup
Flash Point(C)
15 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Nonacidic nitration of secondary amines
Bottaro JC, et al.
The Journal of Organic Chemistry, 52.11, 2292-2294 (1987)
Comparison of synthesis yields of 3'-deoxy-3'-[18F] fluorothymidine by nucleophilic fluorination in various alcohol solvents
Lee SJ, et al.
Journal of Labelled Compounds & Radiopharmaceuticals, 51.1, 80-82 (2008)
Mechanistic studies on gas-phase negative ion unimolecular decompositions. Alkoxide anions
Tumas W, et al.
Journal of the American Chemical Society, 110.9 , 2714-2722 (1988)
The Preparation and Some Reactions of Trifluorovinyllithium1
Tarrant P, et al.
The Journal of Organic Chemistry, 28.3, 839-843 (1963)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service